Please Visit our new Website here
Learn more at http://skmclasses.weebly.com/iit-jee-home-tuitions-bangalore.html
search for videos in http://skmclasses.weebly.com
Twitter – https://twitter.com/ZookeeperPhy
Facebook – https://www.facebook.com/IIT.JEE.by.Prof.Subhashish/
Blog – http://skmclasses.kinja.com
Chemistry Physics Mathematics personal tuitions ( also Home Tuitions / Coaching by Home Tutor with personal Attention ) are available in the Bannerghatta Road IIM ( south Bangalore ) region.
Contact mokshya@gmail.com
–
Students staying in J P Nagar, Bommanahalli, Nayak Layout, Poornima Nagar, Aradhana Layout, Shreyas Colony, Devarachikkahalli, Rukmaiah Layout, Viswapriya Nagar, Akshayanagar, Omkar Nagar, BTM, Shanthiniketan Layout, Madivala, Teacher’s Colony, Hogasandra, MICO Layout, Fortis Hospital, Anjanadri Layout, Apollo Hospital, Royal Lake Front, Royal Residency, Jayanagar, Vijaya Enclave, Sundaram Shetty Nagar, Duo Heights, Arekere, Begur Road, L&T South city, Dollar colony, Brigade Millennium, Kumaraswami Layout, Jarganahalli, Bendre Nagar, Srinidhi Layout, Mysore Bank Colony, Ramaiah Garden, Nobo Nagar, Adigas Restaurant Bannerghatta Road, Shankranthi Layout, Sarvabhouma Nagar, BTS Layout, Ayyappa Temple Shobha Apartments, Anugraha Layout, Neo Layout, Mahaveer Rhyolities, Akshaya Nagar, DLF Extention, new Dollar Colony, etc can easily access this.
The schools and Institutions near by are Mitra Academy, St Pauls – Presidency School and College, Clarence High School, PSBB, Ryan International School, Sarala Birla Academy, BGS NPS,Brigade school, Shantiniketan, MG Infant, Deeksha Hosur Road, Nightingles English Highschool, Sri Venkateshwara Education Society, Oxford Engineering College, Lorven International Institute, Hill Top School, Karnataka Govt. High School, Christ Academy Hulahalli Koppa Road, Salonee School, Royal Convent School, St Francis School, Teresa Public School, Maaruthi Magnolia etc.
Must see https://zookeepersblog.wordpress.com/some-points-which-i-wish-all-my-new-prospective-students-know/
e-Book-e-Book-e-Book-e-Book-e-Book-e-Book-e-Book-e-Book–e-Book–e-Book–e-Book
Did you like the Facebook Page of SKMClasses ?
Please Like the page https://www.facebook.com/IIT.JEE.by.Prof.Subhashish/
Did you send Friend request to Professor Subhashish Chattopadhyay ?
Please first send friend request to profile https://www.facebook.com/Subhashish.SKMClasses
You are allowed to download books only after liking the SKMClasses Page and sending friend request to Subhashish Sir.
Download the following FREE pdf e-Books ( Chapter wise / Topic wise solutions, Written by Prof. Subhashish Chattopadhyay SKMClasses Bangalore )
Everything can be searched in the net. Someone can know and find; if he wants to. Yet information at a single place helps. In fact organized information communicates much better. For IIT results analysis, performance of Girls, perceptions and priorities of various people in various regions of India see
IIT JEE Results and the situation in Bangalore by Prof. Subhashish ( Download )
( Click on the links to open the PDF in a new tab, then Save / Download )
IIT-JEE, NCERT / CBSE, I.Sc., PU, Board exam, EAMCET, BITS Physics Books with lots of Examples ( Free pdf download of Physics Books, Chapter wise / Topic wise Questions and Solutions )
27 ] CBSE & IIT-JEE Physics Survival Guide – Thermal Properties of Solids, or Thermal Properties of Material, Thermal Conductivity etc – by Professor Subhashish Chattopadhyay, Bangalore.
Description – “ Spoon Feeding CBSE & IIT-JEE Physics Survival Guide – Thermal Properties of Solids, or Thermal Properties of Material, Thermal Conductivity Various Methods by Professor Subhashish Chattopadhyay ” for IIT-JEE, I.Sc., CBSE, Karnataka PU, State Boards etc. CBSE Standard 12 and IIT-JEE Physics Survival Guide – CBSE & IIT-JEE Physics Survival Guide – Thermal Properties of Solids, or Thermal Properties of Material, Thermal Conductivity Various Methods etc by Professor Subhashish Chattopadhyay SKMClasses Bangalore Useful for I.Sc. PU-II COMED-K CEE IGCSE IB AP-Chemistry, CET, VIT, Manipal, SRM and other exams.
This e-Book covers several examples of Thermal Properties of Solids, or Thermal Properties of Material, Thermal Conductivity Various Methods etc are also covered. There are many kinds of Problems which are NOT covered in Professor H C Verma ‘s books ( Concepts of Physics ) or Irodov, or ” Resnick & Halliday “. Some examples being split Lenses, Fresnel’s Biprism, Polytropic Processes, Silvered lenses, Slab with a lens like hole or filled with liquids, Cylindrical lenses, isodiaphers, Spallation Reaction, Magic Numbers, Doubly Magic Numbers, Metamaterials with Negative Refractive Index etc. All these kinds of Questions which have been asked in various exams are covered in eBooks of Professor Subhashish Chattopadhyay. Several Complicated examples and many more, and various incomplete dictionary kinds of collection for Course of IIT-JEE, CET, COMED-K etc with CBSE, CEE, IIT-JEE ( Main and Advanced ) Problems and Solutions. Includes NCERT / CBSE Text Book Solutions, Chapter wise Solutions, AIEEE ( Now known as IIT-JEE main ) Solutions, Roorkey Entrance Exam Solutions, EAMCET Solutions.
cbse-iit-jee-physics-survival-guide-thermal-properties-of-solids-by-prof-subhashish-chattopadhyay
:-{D
26 ] CBSE & IIT-JEE Physics Survival Guide – Buoyant Force, Buoyancy, Discussions on Layer of Liquid below the Object etc – by Professor Subhashish Chattopadhyay, Bangalore.
Description – “ Spoon Feeding CBSE & IIT-JEE Physics Survival Guide – Buoyant Force, Buoyancy, Discussions on Layer of Liquid below the Object Various Methods by Professor Subhashish Chattopadhyay ” for IIT-JEE, I.Sc., CBSE, Karnataka PU, State Boards etc. CBSE Standard 12 and IIT-JEE Physics Survival Guide – CBSE & IIT-JEE Physics Survival Guide – Buoyant Force, Buoyancy, Discussions on Layer of Liquid below the Object Various Methods etc by Professor Subhashish Chattopadhyay SKMClasses Bangalore Useful for I.Sc. PU-II COMED-K CEE IGCSE IB AP-Chemistry, CET, VIT, Manipal, SRM and other exams.
This e-Book covers several examples of Buoyant Force, Buoyancy, Discussions on Layer of Liquid below the Object Various Methods etc are also covered. There are many kinds of Problems which are NOT covered in Professor H C Verma ‘s books ( Concepts of Physics ) or Irodov, or ” Resnick & Halliday “. Some examples being split Lenses, Fresnel’s Biprism, Polytropic Processes, Silvered lenses, Slab with a lens like hole or filled with liquids, Cylindrical lenses, isodiaphers, Spallation Reaction, Magic Numbers, Doubly Magic Numbers, Metamaterials with Negative Refractive Index etc. All these kinds of Questions which have been asked in various exams are covered in eBooks of Professor Subhashish Chattopadhyay. Several Complicated examples and many more, and various incomplete dictionary kinds of collection for Course of IIT-JEE, CET, COMED-K etc with CBSE, CEE, IIT-JEE ( Main and Advanced ) Problems and Solutions. Includes NCERT / CBSE Text Book Solutions, Chapter wise Solutions, AIEEE ( Now known as IIT-JEE main ) Solutions, Roorkey Entrance Exam Solutions, EAMCET Solutions.
cbse-iit-jee-physics-survival-guide-buoyant-force-by-prof-subhashish-chattopadhyay
:-{D
25 ] CBSE & IIT-JEE Physics Survival Guide – Mechanical Properties of Material, Mechanical Properties of Solids, Young ‘s Modulus, Bulk Modulus, Poisson ‘s Ratio, Shear Stress, Strain, Energy Stored in elongated wire etc – by Professor Subhashish Chattopadhyay, Bangalore.
Description – “ Spoon Feeding CBSE & IIT-JEE Physics Survival Guide – Mechanical Properties of Material, Mechanical Properties of Solids, Young ‘s Modulus, Bulk Modulus, Poisson ‘s Ratio, Shear Stress, Strain, Energy Stored in elongated wire Various Methods by Professor Subhashish Chattopadhyay ” for IIT-JEE, I.Sc., CBSE, Karnataka PU, State Boards etc. CBSE Standard 12 and IIT-JEE Physics Survival Guide – CBSE & IIT-JEE Physics Survival Guide – Mechanical Properties of Material, Mechanical Properties of Solids, Young ‘s Modulus, Bulk Modulus, Poisson ‘s Ratio, Shear Stress, Strain, Energy Stored in elongated wire Various Methods etc by Professor Subhashish Chattopadhyay SKMClasses Bangalore Useful for I.Sc. PU-II COMED-K CEE IGCSE IB AP-Chemistry, CET, VIT, Manipal, SRM and other exams.
This e-Book covers several examples of Mechanical Properties of Material, Mechanical Properties of Solids, Young ‘s Modulus, Bulk Modulus, Poisson ‘s Ratio, Shear Stress, Strain, Energy Stored in elongated wire Various Methods etc are also covered. There are many kinds of Problems which are NOT covered in Professor H C Verma ‘s books ( Concepts of Physics ) or Irodov, or ” Resnick & Halliday “. Some examples being split Lenses, Fresnel’s Biprism, Polytropic Processes, Silvered lenses, Slab with a lens like hole or filled with liquids, Cylindrical lenses, isodiaphers, Spallation Reaction, Magic Numbers, Doubly Magic Numbers, Metamaterials with Negative Refractive Index etc. All these kinds of Questions which have been asked in various exams are covered in eBooks of Professor Subhashish Chattopadhyay. Several Complicated examples and many more, and various incomplete dictionary kinds of collection for Course of IIT-JEE, CET, COMED-K etc with CBSE, CEE, IIT-JEE ( Main and Advanced ) Problems and Solutions. Includes NCERT / CBSE Text Book Solutions, Chapter wise Solutions, AIEEE ( Now known as IIT-JEE main ) Solutions, Roorkey Entrance Exam Solutions, EAMCET Solutions.
cbse-iit-jee-physics-survival-guide-mechanical-properties-of-solids-by-prof-subhashish
:-{D
24 ] CBSE & IIT-JEE Physics Survival Guide – Kinetic Theory of Gases etc – by Professor Subhashish Chattopadhyay, Bangalore.
Description – “ Spoon Feeding CBSE & IIT-JEE Physics Survival Guide – Kinetic Theory of Gases Various Methods by Professor Subhashish Chattopadhyay ” for IIT-JEE, I.Sc., CBSE, Karnataka PU, State Boards etc. CBSE Standard 12 and IIT-JEE Physics Survival Guide – CBSE & IIT-JEE Physics Survival Guide – Kinetic Theory of Gases Various Methods etc by Professor Subhashish Chattopadhyay SKMClasses Bangalore Useful for I.Sc. PU-II COMED-K CEE IGCSE IB AP-Chemistry, CET, VIT, Manipal, SRM and other exams.
This e-Book covers several examples of Kinetic Theory of Gases Various Methods etc are also covered. There are many kinds of Problems which are NOT covered in Professor H C Verma ‘s books ( Concepts of Physics ) or Irodov, or ” Resnick & Halliday “. Some examples being split Lenses, Fresnel’s Biprism, Polytropic Processes, Silvered lenses, Slab with a lens like hole or filled with liquids, Cylindrical lenses, isodiaphers, Spallation Reaction, Magic Numbers, Doubly Magic Numbers, Metamaterials with Negative Refractive Index etc. All these kinds of Questions which have been asked in various exams are covered in eBooks of Professor Subhashish Chattopadhyay. Several Complicated examples and many more, and various incomplete dictionary kinds of collection for Course of IIT-JEE, CET, COMED-K etc with CBSE, CEE, IIT-JEE ( Main and Advanced ) Problems and Solutions. Includes NCERT / CBSE Text Book Solutions, Chapter wise Solutions, AIEEE ( Now known as IIT-JEE main ) Solutions, Roorkey Entrance Exam Solutions, EAMCET Solutions.
cbse-iit-jee-physics-survival-guide-kinetic-theory-of-gasess-by-prof-subhashish-chattopadhyay
:-{D
23 ] CBSE & IIT-JEE Physics Survival Guide – Vectors & Scalars etc – by Professor Subhashish Chattopadhyay, Bangalore.
Description – “ Spoon Feeding CBSE & IIT-JEE Physics Survival Guide – Vectors & Scalars Various Methods by Professor Subhashish Chattopadhyay ” for IIT-JEE, I.Sc., CBSE, Karnataka PU, State Boards etc. CBSE Standard 12 and IIT-JEE Physics Survival Guide – CBSE & IIT-JEE Physics Survival Guide – Vectors & Scalars Various Methods etc by Professor Subhashish Chattopadhyay SKMClasses Bangalore Useful for I.Sc. PU-II COMED-K CEE IGCSE IB AP-Chemistry, CET, VIT, Manipal, SRM and other exams.
This e-Book covers several examples of Vectors & Scalars Various Methods etc are also covered. There are many kinds of Problems which are NOT covered in Professor H C Verma ‘s books ( Concepts of Physics ) or Irodov, or ” Resnick & Halliday “. Some examples being split Lenses, Fresnel’s Biprism, Polytropic Processes, Silvered lenses, Slab with a lens like hole or filled with liquids, Cylindrical lenses, isodiaphers, Spallation Reaction, Magic Numbers, Doubly Magic Numbers, Metamaterials with Negative Refractive Index etc. All these kinds of Questions which have been asked in various exams are covered in eBooks of Professor Subhashish Chattopadhyay. Several Complicated examples and many more, and various incomplete dictionary kinds of collection for Course of IIT-JEE, CET, COMED-K etc with CBSE, CEE, IIT-JEE ( Main and Advanced ) Problems and Solutions. Includes NCERT / CBSE Text Book Solutions, Chapter wise Solutions, AIEEE ( Now known as IIT-JEE main ) Solutions, Roorkey Entrance Exam Solutions, EAMCET Solutions.
cbse-iit-jee-physics-survival-guide-vectors-scalars-by-prof-subhashish-chattopadhyay
:-{D
22 ] CBSE & IIT-JEE Physics Survival Guide – Units, Dimensions, Measurements & Errors etc – by Professor Subhashish Chattopadhyay, Bangalore.
Description – “ Spoon Feeding CBSE & IIT-JEE Physics Survival Guide – Units, Dimensions, Measurements & Errors Various Methods by Professor Subhashish Chattopadhyay ” for IIT-JEE, I.Sc., CBSE, Karnataka PU, State Boards etc. CBSE Standard 12 and IIT-JEE Physics Survival Guide – CBSE & IIT-JEE Physics Survival Guide – Units, Dimensions, Measurements & Errors Various Methods etc by Professor Subhashish Chattopadhyay SKMClasses Bangalore Useful for I.Sc. PU-II COMED-K CEE IGCSE IB AP-Chemistry, CET, VIT, Manipal, SRM and other exams.
This e-Book covers several examples of Units, Dimensions, Measurements & Errors Various Methods etc are also covered. There are many kinds of Problems which are NOT covered in Professor H C Verma ‘s books ( Concepts of Physics ) or Irodov, or ” Resnick & Halliday “. Some examples being split Lenses, Fresnel’s Biprism, Polytropic Processes, Silvered lenses, Slab with a lens like hole or filled with liquids, Cylindrical lenses, isodiaphers, Spallation Reaction, Magic Numbers, Doubly Magic Numbers, Metamaterials with Negative Refractive Index etc. All these kinds of Questions which have been asked in various exams are covered in eBooks of Professor Subhashish Chattopadhyay. Several Complicated examples and many more, and various incomplete dictionary kinds of collection for Course of IIT-JEE, CET, COMED-K etc with CBSE, CEE, IIT-JEE ( Main and Advanced ) Problems and Solutions. Includes NCERT / CBSE Text Book Solutions, Chapter wise Solutions, AIEEE ( Now known as IIT-JEE main ) Solutions, Roorkey Entrance Exam Solutions, EAMCET Solutions.
cbse-iit-jee-physics-survival-guide-units-dimensions-by-prof-subhashish-chattopadhyay
:-{D
21 ] CBSE & IIT-JEE Physics Survival Guide – Kinematics, Dynamics or Kinetics, Circular Motion & Projectile Motion etc – by Professor Subhashish Chattopadhyay, Bangalore.
Description – “ Spoon Feeding CBSE & IIT-JEE Physics Survival Guide – Kinematics, Dynamics or Kinetics, Circular Motion & Projectile Motion Various Methods by Professor Subhashish Chattopadhyay ” for IIT-JEE, I.Sc., CBSE, Karnataka PU, State Boards etc. CBSE Standard 12 and IIT-JEE Physics Survival Guide – CBSE & IIT-JEE Physics Survival Guide – Kinematics, Dynamics or Kinetics, Circular Motion & Projectile Motion Various Methods etc by Professor Subhashish Chattopadhyay SKMClasses Bangalore Useful for I.Sc. PU-II COMED-K CEE IGCSE IB AP-Chemistry, CET, VIT, Manipal, SRM and other exams.
This e-Book covers several examples of Kinematics, Dynamics or Kinetics, Circular Motion & Projectile Motion, Various Methods etc are also covered. There are many kinds of Problems which are NOT covered in Professor H C Verma ‘s books ( Concepts of Physics ) or Irodov, or ” Resnick & Halliday “. Some examples being split Lenses, Fresnel’s Biprism, Polytropic Processes, Silvered lenses, Slab with a lens like hole or filled with liquids, Cylindrical lenses, isodiaphers, Spallation Reaction, Magic Numbers, Doubly Magic Numbers, Metamaterials with Negative Refractive Index etc. All these kinds of Questions which have been asked in various exams are covered in eBooks of Professor Subhashish Chattopadhyay. Several Complicated examples and many more, and various incomplete dictionary kinds of collection for Course of IIT-JEE, CET, COMED-K etc with CBSE, CEE, IIT-JEE ( Main and Advanced ) Problems and Solutions. Includes NCERT / CBSE Text Book Solutions, Chapter wise Solutions, AIEEE ( Now known as IIT-JEE main ) Solutions, Roorkey Entrance Exam Solutions, EAMCET Solutions.
cbse-iit-jee-physics-survival-guide-kinematics-by-prof-subhashish-chattopadhyay
:-{D
20 ] CBSE & IIT-JEE Physics Survival Guide – Measuring Speed of Light, Various Methods etc – by Professor Subhashish Chattopadhyay, Bangalore.
Description – “ Spoon Feeding CBSE & IIT-JEE Physics Survival Guide – Measuring Speed of Light, Various Methods by Professor Subhashish Chattopadhyay ” for IIT-JEE, I.Sc., CBSE, Karnataka PU, State Boards etc. CBSE Standard 12 and IIT-JEE Physics Survival Guide – CBSE & IIT-JEE Physics Survival Guide – Measuring Speed of Light, Various Methods etc by Professor Subhashish Chattopadhyay SKMClasses Bangalore Useful for I.Sc. PU-II COMED-K CEE IGCSE IB AP-Chemistry, CET, VIT, Manipal, SRM and other exams.
This e-Book covers several examples of Measuring Speed of Light, Various Methods etc are also covered. There are many kinds of Problems which are NOT covered in Professor H C Verma ‘s books ( Concepts of Physics ) or Irodov, or ” Resnick & Halliday “. Some examples being split Lenses, Fresnel’s Biprism, Polytropic Processes, Silvered lenses, Slab with a lens like hole or filled with liquids, Cylindrical lenses, isodiaphers, Spallation Reaction, Magic Numbers, Doubly Magic Numbers, Metamaterials with Negative Refractive Index etc. All these kinds of Questions which have been asked in various exams are covered in eBooks of Professor Subhashish Chattopadhyay. Several Complicated examples and many more, and various incomplete dictionary kinds of collection for Course of IIT-JEE, CET, COMED-K etc with CBSE, CEE, IIT-JEE ( Main and Advanced ) Problems and Solutions. Includes NCERT / CBSE Text Book Solutions, Chapter wise Solutions, AIEEE ( Now known as IIT-JEE main ) Solutions, Roorkey Entrance Exam Solutions, EAMCET Solutions.
cbse-iit-jee-physics-survival-guide-speed-of-light-by-prof-subhashish-chattopadhyay
:-{D
19 ] CBSE & IIT-JEE Physics Survival Guide – Maxwell ‘s Equations, Electromagnetic Waves etc – by Professor Subhashish Chattopadhyay, Bangalore.
Description – “ Spoon Feeding CBSE & IIT-JEE Physics Survival Guide – Maxwell ‘s Equations, Electromagnetic Waves by Professor Subhashish Chattopadhyay ” for IIT-JEE, I.Sc., CBSE, Karnataka PU, State Boards etc. CBSE Standard 12 and IIT-JEE Physics Survival Guide – CBSE & IIT-JEE Physics Survival Guide – Maxwell ‘s Equations, Electromagnetic Waves etc by Professor Subhashish Chattopadhyay SKMClasses Bangalore Useful for I.Sc. PU-II COMED-K CEE IGCSE IB AP-Chemistry, CET, VIT, Manipal, SRM and other exams.
This e-Book covers several examples of Maxwell ‘s Equations & Electromagnetic Waves etc are also covered. There are many kinds of Problems which are NOT covered in Professor H C Verma ‘s books ( Concepts of Physics ) or Irodov, or ” Resnick & Halliday “. Some examples being split Lenses, Fresnel’s Biprism, Polytropic Processes, Silvered lenses, Slab with a lens like hole or filled with liquids, Cylindrical lenses, isodiaphers, Spallation Reaction, Magic Numbers, Doubly Magic Numbers, Metamaterials with Negative Refractive Index etc. All these kinds of Questions which have been asked in various exams are covered in eBooks of Professor Subhashish Chattopadhyay. Several Complicated examples and many more, and various incomplete dictionary kinds of collection for Course of IIT-JEE, CET, COMED-K etc with CBSE, CEE, IIT-JEE ( Main and Advanced ) Problems and Solutions. Includes NCERT / CBSE Text Book Solutions, Chapter wise Solutions, AIEEE ( Now known as IIT-JEE main ) Solutions, Roorkey Entrance Exam Solutions, EAMCET Solutions.
cbse-iit-jee-physics-survival-guide-maxwells-equations-electromagnetic-waves-by-prof-subhashish
:-{D
18 ] CBSE & IIT-JEE Physics Survival Guide – Magnetism History etc – by Professor Subhashish Chattopadhyay, Bangalore.
Description – “ Spoon Feeding CBSE & IIT-JEE Physics Survival Guide – Magnetism History by Professor Subhashish Chattopadhyay ” for IIT-JEE, I.Sc., CBSE, Karnataka PU, State Boards etc. CBSE Standard 12 and IIT-JEE Physics Survival Guide – CBSE & IIT-JEE Physics Survival Guide – Magnetism History etc by Professor Subhashish Chattopadhyay SKMClasses Bangalore Useful for I.Sc. PU-II COMED-K CEE IGCSE IB AP-Chemistry, CET, VIT, Manipal, SRM and other exams.
This e-Book covers several examples of Magnetism History etc are also covered. There are many kinds of Problems which are NOT covered in Professor H C Verma ‘s books ( Concepts of Physics ) or Irodov, or ” Resnick & Halliday “. Some examples being split Lenses, Fresnel’s Biprism, Polytropic Processes, Silvered lenses, Slab with a lens like hole or filled with liquids, Cylindrical lenses, isodiaphers, Spallation Reaction, Magic Numbers, Doubly Magic Numbers, Metamaterials with Negative Refractive Index etc. All these kinds of Questions which have been asked in various exams are covered in eBooks of Professor Subhashish Chattopadhyay. Several Complicated examples and many more, and various incomplete dictionary kinds of collection for Course of IIT-JEE, CET, COMED-K etc with CBSE, CEE, IIT-JEE ( Main and Advanced ) Problems and Solutions. Includes NCERT / CBSE Text Book Solutions, Chapter wise Solutions, AIEEE ( Now known as IIT-JEE main ) Solutions, Roorkey Entrance Exam Solutions, EAMCET Solutions.
cbse-iit-jee-physics-survival-guide-magnetism-by-prof-subhashish-chattopadhyay
:-{D
17 ] CBSE & IIT-JEE Physics Survival Guide – Magnetic Induction, Voltage Produced etc – by Professor Subhashish Chattopadhyay, Bangalore.
Description – “ Spoon Feeding CBSE & IIT-JEE Physics Survival Guide – Magnetic Induction, Voltage Produced by Professor Subhashish Chattopadhyay ” for IIT-JEE, I.Sc., CBSE, Karnataka PU, State Boards etc. CBSE Standard 12 and IIT-JEE Physics Survival Guide – CBSE & IIT-JEE Physics Survival Guide – Magnetic Induction, Voltage Produced by Professor Subhashish Chattopadhyay SKMClasses Bangalore Useful for I.Sc. PU-II COMED-K CEE IGCSE IB AP-Chemistry, CET, VIT, Manipal, SRM and other exams.
This e-Book covers several examples of Magnetic Induction, Voltage Produced etc are also covered. There are many kinds of Problems which are NOT covered in Professor H C Verma ‘s books ( Concepts of Physics ) or Irodov, or ” Resnick & Halliday “. Some examples being split Lenses, Fresnel’s Biprism, Polytropic Processes, Silvered lenses, Slab with a lens like hole or filled with liquids, Cylindrical lenses, isodiaphers, Spallation Reaction, Magic Numbers, Doubly Magic Numbers, Metamaterials with Negative Refractive Index etc. All these kinds of Questions which have been asked in various exams are covered in eBooks of Professor Subhashish Chattopadhyay. Several Complicated examples and many more, and various incomplete dictionary kinds of collection for Course of IIT-JEE, CET, COMED-K etc with CBSE, CEE, IIT-JEE ( Main and Advanced ) Problems and Solutions. Includes NCERT / CBSE Text Book Solutions, Chapter wise Solutions, AIEEE ( Now known as IIT-JEE main ) Solutions, Roorkey Entrance Exam Solutions, EAMCET Solutions.
cbse-iit-jee-physics-survival-guide-magnetic-induction-by-prof-subhashish-chattopadhyay
:-{D
16 ] CBSE & IIT-JEE Physics Survival Guide – Magnetic Effects of Current etc – by Professor Subhashish Chattopadhyay, Bangalore.
Description – “ Spoon Feeding CBSE & IIT-JEE Physics Survival Guide – Magnetic Effects of Current by Professor Subhashish Chattopadhyay ” for IIT-JEE, I.Sc., CBSE, Karnataka PU, State Boards etc. CBSE Standard 12 and IIT-JEE Physics Survival Guide – CBSE & IIT-JEE Physics Survival Guide – Magnetic Effects of Current by Professor Subhashish Chattopadhyay SKMClasses Bangalore Useful for I.Sc. PU-II COMED-K CEE IGCSE IB AP-Chemistry, CET, VIT, Manipal, SRM and other exams.
This e-Book covers several examples of Magnetic Effects of Current, Various Derivations etc are also covered. There are many kinds of Problems which are NOT covered in Professor H C Verma ‘s books ( Concepts of Physics ) or Irodov, or ” Resnick & Halliday “. Some examples being split Lenses, Fresnel’s Biprism, Polytropic Processes, Silvered lenses, Slab with a lens like hole or filled with liquids, Cylindrical lenses, isodiaphers, Spallation Reaction, Magic Numbers, Doubly Magic Numbers, Metamaterials with Negative Refractive Index etc. All these kinds of Questions which have been asked in various exams are covered in eBooks of Professor Subhashish Chattopadhyay. Several Complicated examples and many more, and various incomplete dictionary kinds of collection for Course of IIT-JEE, CET, COMED-K etc with CBSE, CEE, IIT-JEE ( Main and Advanced ) Problems and Solutions. Includes NCERT / CBSE Text Book Solutions, Chapter wise Solutions, AIEEE ( Now known as IIT-JEE main ) Solutions, Roorkey Entrance Exam Solutions, EAMCET Solutions.
cbse-iit-jee-physics-survival-guide-magnetic-effect-of-current-by-prof-subhashish-chattopadhyay
:-{D
15 ] CBSE & IIT-JEE Physics Survival Guide – Capacitance Dielectrics & Circuits etc – by Professor Subhashish Chattopadhyay, Bangalore.
Description – “ Spoon Feeding CBSE & IIT-JEE Physics Survival Guide – Capacitance Dielectrics & Circuits by Professor Subhashish Chattopadhyay ” for IIT-JEE, I.Sc., CBSE, Karnataka PU, State Boards etc. CBSE Standard 12 and IIT-JEE Physics Survival Guide – CBSE & IIT-JEE Physics Survival Guide – Capacitance Dielectrics & Circuits by Professor Subhashish Chattopadhyay SKMClasses Bangalore Useful for I.Sc. PU-II COMED-K CEE IGCSE IB AP-Chemistry, CET, VIT, Manipal, SRM and other exams.
This e-Book covers several examples of Capacitance, Trick Circuits, Combinations of Dielectrics, Various Derivations etc are also covered. There are many kinds of Problems which are NOT covered in Professor H C Verma ‘s books ( Concepts of Physics ) or Irodov, or ” Resnick & Halliday “. Some examples being split Lenses, Fresnel’s Biprism, Polytropic Processes, Silvered lenses, Slab with a lens like hole or filled with liquids, Cylindrical lenses, isodiaphers, Spallation Reaction, Magic Numbers, Doubly Magic Numbers, Metamaterials with Negative Refractive Index etc. All these kinds of Questions which have been asked in various exams are covered in eBooks of Professor Subhashish Chattopadhyay. Several Complicated examples and many more, and various incomplete dictionary kinds of collection for Course of IIT-JEE, CET, COMED-K etc with CBSE, CEE, IIT-JEE ( Main and Advanced ) Problems and Solutions. Includes NCERT / CBSE Text Book Solutions, Chapter wise Solutions, AIEEE ( Now known as IIT-JEE main ) Solutions, Roorkey Entrance Exam Solutions, EAMCET Solutions.
cbse-iit-jee-physics-survival-guide-capacitance-by-prof-subhashish-chattopadhyay
:-{D
14 ] CBSE & IIT-JEE Physics Survival Guide – Electrostatics & Gauss Theorem etc – by Professor Subhashish Chattopadhyay, Bangalore.
Description – “ Spoon Feeding CBSE & IIT-JEE Physics Survival Guide – Electrostatics & Gauss Theorem by Professor Subhashish Chattopadhyay ” for IIT-JEE, I.Sc., CBSE, Karnataka PU, State Boards etc. CBSE Standard 12 and IIT-JEE Physics Survival Guide – CBSE & IIT-JEE Physics Survival Guide – Electrostatics & Gauss Theorem by Professor Subhashish Chattopadhyay SKMClasses Bangalore Useful for I.Sc. PU-II COMED-K CEE IGCSE IB AP-Chemistry, CET, VIT, Manipal, SRM and other exams.
This e-Book covers several examples of Electrostatics, Gauss Theorem, Various Derivations etc are also covered. There are many kinds of Problems which are NOT covered in Professor H C Verma ‘s books ( Concepts of Physics ) or Irodov, or ” Resnick & Halliday “. Some examples being split Lenses, Fresnel’s Biprism, Polytropic Processes, Silvered lenses, Slab with a lens like hole or filled with liquids, Cylindrical lenses, isodiaphers, Spallation Reaction, Magic Numbers, Doubly Magic Numbers, Metamaterials with Negative Refractive Index etc. All these kinds of Questions which have been asked in various exams are covered in eBooks of Professor Subhashish Chattopadhyay. Several Complicated examples and many more, and various incomplete dictionary kinds of collection for Course of IIT-JEE, CET, COMED-K etc with CBSE, CEE, IIT-JEE ( Main and Advanced ) Problems and Solutions. Includes NCERT / CBSE Text Book Solutions, Chapter wise Solutions, AIEEE ( Now known as IIT-JEE main ) Solutions, Roorkey Entrance Exam Solutions, EAMCET Solutions.
cbse-iit-jee-physics-survival-guide-electrostatics-gauss-theorem-by-prof-subhashish-chattopadhyay
:-{D
13 ] CBSE & IIT-JEE Physics Survival Guide – Center of Mass etc – by Professor Subhashish Chattopadhyay, Bangalore.
Description – “ Spoon Feeding CBSE & IIT-JEE Physics Survival Guide – Center of Mass by Professor Subhashish Chattopadhyay ” for IIT-JEE, I.Sc., CBSE, Karnataka PU, State Boards etc. CBSE Standard 12 and IIT-JEE Physics Survival Guide – CBSE & IIT-JEE Physics Survival Guide – Center of Mass by Professor Subhashish Chattopadhyay SKMClasses Bangalore Useful for I.Sc. PU-II COMED-K CEE IGCSE IB AP-Chemistry, CET, VIT, Manipal, SRM and other exams.
This e-Book covers several examples of Center of Mass, Various Derivations etc are also covered. There are many kinds of Problems which are NOT covered in Professor H C Verma ‘s books ( Concepts of Physics ) or Irodov, or ” Resnick & Halliday “. Some examples being split Lenses, Fresnel’s Biprism, Polytropic Processes, Silvered lenses, Slab with a lens like hole or filled with liquids, Cylindrical lenses, isodiaphers, Spallation Reaction, Magic Numbers, Doubly Magic Numbers, Metamaterials with Negative Refractive Index etc. All these kinds of Questions which have been asked in various exams are covered in eBooks of Professor Subhashish Chattopadhyay. Several Complicated examples and many more, and various incomplete dictionary kinds of collection for Course of IIT-JEE, CET, COMED-K etc with CBSE, CEE, IIT-JEE ( Main and Advanced ) Problems and Solutions. Includes NCERT / CBSE Text Book Solutions, Chapter wise Solutions, AIEEE ( Now known as IIT-JEE main ) Solutions, Roorkey Entrance Exam Solutions, EAMCET Solutions.
cbse-iit-jee-physics-survival-guide-center-of-mass-by-prof-subhashish-chattopadhyay
:-{D
12 ] CBSE & IIT-JEE Physics Survival Guide – Work Power Energy Variable Force Leaking Bucket etc – by Professor Subhashish Chattopadhyay, Bangalore.
Description – “ Spoon Feeding CBSE & IIT-JEE Physics Survival Guide – Work Power Energy Variable Force Leaking Bucket by Professor Subhashish Chattopadhyay ” for IIT-JEE, I.Sc., CBSE, Karnataka PU, State Boards etc. CBSE Standard 12 and IIT-JEE Physics Survival Guide – CBSE & IIT-JEE Physics Survival Guide – Work Power Energy Variable Force Leaking Bucket by Professor Subhashish Chattopadhyay SKMClasses Bangalore Useful for I.Sc. PU-II COMED-K CEE IGCSE IB AP-Chemistry, CET, VIT, Manipal, SRM and other exams.
This e-Book covers several examples of Work Power Energy Variable Force Leaking Bucket, Various Derivations etc are also covered. There are many kinds of Problems which are NOT covered in Professor H C Verma ‘s books ( Concepts of Physics ) or Irodov, or ” Resnick & Halliday “. Some examples being split Lenses, Fresnel’s Biprism, Polytropic Processes, Silvered lenses, Slab with a lens like hole or filled with liquids, Cylindrical lenses, isodiaphers, Spallation Reaction, Magic Numbers, Doubly Magic Numbers, Metamaterials with Negative Refractive Index etc. All these kinds of Questions which have been asked in various exams are covered in eBooks of Professor Subhashish Chattopadhyay. Several Complicated examples and many more, and various incomplete dictionary kinds of collection for Course of IIT-JEE, CET, COMED-K etc with CBSE, CEE, IIT-JEE ( Main and Advanced ) Problems and Solutions. Includes NCERT / CBSE Text Book Solutions, Chapter wise Solutions, AIEEE ( Now known as IIT-JEE main ) Solutions, Roorkey Entrance Exam Solutions, EAMCET Solutions.
cbse-iit-jee-physics-survival-guide-work-power-energy-by-prof-subhashish-chattopadhyay
:-{D
11 ] CBSE & IIT-JEE Physics Survival Guide – Moment of Inertia, Solid Bodies Angular Momentum etc – by Professor Subhashish Chattopadhyay, Bangalore.
Description – “ Spoon Feeding CBSE & IIT-JEE Physics Survival Guide – Moment of Inertia of Solid Bodies by Professor Subhashish Chattopadhyay ” for IIT-JEE, I.Sc., CBSE, Karnataka PU, State Boards etc. CBSE Standard 12 and IIT-JEE Physics Survival Guide – CBSE & IIT-JEE Physics Survival Guide – Moment of Inertia of Solid Bodies by Professor Subhashish Chattopadhyay SKMClasses Bangalore Useful for I.Sc. PU-II COMED-K CEE IGCSE IB AP-Chemistry, CET, VIT, Manipal, SRM and other exams.
This e-Book covers several examples of Moment of Inertia, Solid Bodies Angular Momentum, Rotational Energy, Derivations etc are also covered. There are many kinds of Problems which are NOT covered in Professor H C Verma ‘s books ( Concepts of Physics ) or Irodov, or ” Resnick & Halliday “. Some examples being split Lenses, Fresnel’s Biprism, Polytropic Processes, Silvered lenses, Slab with a lens like hole or filled with liquids, Cylindrical lenses, isodiaphers, Spallation Reaction, Magic Numbers, Doubly Magic Numbers, Metamaterials with Negative Refractive Index etc. All these kinds of Questions which have been asked in various exams are covered in eBooks of Professor Subhashish Chattopadhyay. Several Complicated examples and many more, and various incomplete dictionary kinds of collection for Course of IIT-JEE, CET, COMED-K etc with CBSE, CEE, IIT-JEE ( Main and Advanced ) Problems and Solutions. Includes NCERT / CBSE Text Book Solutions, Chapter wise Solutions, AIEEE ( Now known as IIT-JEE main ) Solutions, Roorkey Entrance Exam Solutions, EAMCET Solutions.
cbse-iit-jee-physics-survival-guide-moment-of-inertia-by-prof-subhashish-chattopadhyay
:-{D
10 ] CBSE & IIT-JEE Physics Survival Guide – Circular Motion, Conical Pendulum etc – by Professor Subhashish Chattopadhyay, Bangalore.
Description – “ Spoon Feeding CBSE & IIT-JEE Physics Survival Guide – Circular Motion, Conical Pendulum etc and many complicated Problems by Professor Subhashish Chattopadhyay ” for IIT-JEE, I.Sc., CBSE, Karnataka PU, State Boards etc. CBSE Standard 12 and IIT-JEE Physics Survival Guide – CBSE & IIT-JEE Physics Survival Guide – Circular Motion by Professor Subhashish Chattopadhyay SKMClasses Bangalore Useful for I.Sc. PU-II COMED-K CEE IGCSE IB AP-Chemistry, CET, VIT, Manipal, SRM and other exams.
This e-Book covers several examples of Circular Motion. Conical Pendulum etc are also covered. There are many kinds of Problems which are NOT covered in Professor H C Verma ‘s books ( Concepts of Physics ) or Irodov, or ” Resnick & Halliday “. Some examples being split Lenses, Fresnel’s Biprism, Polytropic Processes, Silvered lenses, Slab with a lens like hole or filled with liquids, Cylindrical lenses, isodiaphers, Spallation Reaction, Magic Numbers, Doubly Magic Numbers, Metamaterials with Negative Refractive Index etc. All these kinds of Questions which have been asked in various exams are covered in eBooks of Professor Subhashish Chattopadhyay. Several Complicated examples and many more, and various incomplete dictionary kinds of collection for Course of IIT-JEE, CET, COMED-K etc with CBSE, CEE, IIT-JEE ( Main and Advanced ) Problems and Solutions. Includes NCERT / CBSE Text Book Solutions, Chapter wise Solutions, AIEEE ( Now known as IIT-JEE main ) Solutions, Roorkey Entrance Exam Solutions, EAMCET Solutions.
cbse-iit-jee-physics-survival-guide-circular-motion-by-prof-subhashish-chattopadhyay
:-{D
9 ] CBSE & IIT-JEE Physics Survival Guide – Solutions to Irodov Problems, by Subhashish Sir, and Other Professors.
cbse-iit-jee-physics-survival-guide-irodov-problems-solutions-by-prof-subhashish-others
:-{D
8 ] CBSE & IIT-JEE Physics Survival Guide – Electrical Circuits, Delta to Star Conversion, Current Source, Trick Circuits, Unbalanced Wheatstone Bridge, Steps and Techniques of Solving Electrical Circuits etc – by Professor Subhashish Chattopadhyay, Bangalore.
Description – “ Spoon Feeding CBSE & IIT-JEE Physics Survival Guide – Electrical Circuits, Delta to Star Conversion, Current Source, Trick Circuits, Unbalanced Wheatstone Bridge, Steps and Techniques of Solving Electrical Circuits etc and many complicated Problems by Professor Subhashish Chattopadhyay ” for IIT-JEE, I.Sc., CBSE, Karnataka PU, State Boards etc. CBSE Standard 12 and IIT-JEE Physics Survival Guide – CBSE & IIT-JEE Physics Survival Guide – Gravitation by Professor Subhashish Chattopadhyay SKMClasses Bangalore Useful for I.Sc. PU-II COMED-K CEE IGCSE IB AP-Chemistry, CET, VIT, Manipal, SRM and other exams.
This e-Book covers several examples of Electrical Circuits, including Inductance & Capacitance, internal Resistance etc are also covered. There are many kinds of Problems which are NOT covered in Professor H C Verma ‘s books ( Concepts of Physics ) or Irodov, or ” Resnick & Halliday “. Some examples being split Lenses, Fresnel’s Biprism, Polytropic Processes, Silvered lenses, Slab with a lens like hole or filled with liquids, Cylindrical lenses, isodiaphers, Spallation Reaction, Magic Numbers, Doubly Magic Numbers, Metamaterials with Negative Refractive Index etc. All these kinds of Questions which have been asked in various exams are covered in eBooks of Professor Subhashish Chattopadhyay. Several Complicated examples and many more, and various incomplete dictionary kinds of collection for Course of IIT-JEE, CET, COMED-K etc with CBSE, CEE, IIT-JEE ( Main and Advanced ) Problems and Solutions. Includes NCERT / CBSE Text Book Solutions, Chapter wise Solutions, AIEEE ( Now known as IIT-JEE main ) Solutions, Roorkey Entrance Exam Solutions, EAMCET Solutions.
cbse-iit-jee-physics-survival-guide-electrical-circuits-by-prof-subhashish-chattopadhyay
:-{D
7 ] CBSE & IIT-JEE Physics Survival Guide – Gravitation, Contrasting Comparisons of Gravitational Potential and Electrostatic Potential, Contrasting Comparisons of Gravitational Field and Electrostatic Field, Escape Velocity, Height attended by a mass thrown at various speeds etc – by Professor Subhashish Chattopadhyay, Bangalore.
Description – “ Spoon Feeding CBSE & IIT-JEE Physics Survival Guide – Gravitation, Contrasting Comparisons of Gravitational Potential and Electrostatic Potential, Contrasting Comparisons of Gravitational Field and Electrostatic Field, Escape Velocity, Height attended by a mass thrown at various speeds etc and many complicated Problems by Professor Subhashish Chattopadhyay ” for IIT-JEE, I.Sc., CBSE, Karnataka PU, State Boards etc. CBSE Standard 12 and IIT-JEE Physics Survival Guide – CBSE & IIT-JEE Physics Survival Guide – Gravitation by Professor Subhashish Chattopadhyay SKMClasses Bangalore Useful for I.Sc. PU-II COMED-K CEE IGCSE IB AP-Chemistry, CET, VIT, Manipal, SRM and other exams.
This e-Book covers several examples of Gravitation, Field, Potential, escape velocity etc are also covered. There are many kinds of Problems which are NOT covered in Professor H C Verma ‘s books ( Concepts of Physics ) or Irodov, or ” Resnick & Halliday “. Some examples being split Lenses, Fresnel’s Biprism, Polytropic Processes, Silvered lenses, Slab with a lens like hole or filled with liquids, Cylindrical lenses, isodiaphers, Spallation Reaction, Magic Numbers, Doubly Magic Numbers, Metamaterials with Negative Refractive Index etc. All these kinds of Questions which have been asked in various exams are covered in eBooks of Professor Subhashish Chattopadhyay. Several Complicated examples and many more, and various incomplete dictionary kinds of collection for Course of IIT-JEE, CET, COMED-K etc with CBSE, CEE, IIT-JEE ( Main and Advanced ) Problems and Solutions. Includes NCERT / CBSE Text Book Solutions, Chapter wise Solutions, AIEEE ( Now known as IIT-JEE main ) Solutions, Roorkey Entrance Exam Solutions, EAMCET Solutions.
cbse-iit-jee-physics-survival-guide-gravitation-by-prof-subhashish-chattopadhyay
:-{D
6 ] CBSE & IIT-JEE Physics Survival Guide – SHM Periodic Motion, Harmonic Oscillations with Solid Objects, Approximate Simple Harmonic Motions – by Professor Subhashish Chattopadhyay, Bangalore.
Description – “ Spoon Feeding CBSE & IIT-JEE Physics Survival Guide – SHM ( Simple Harmonic Motion ) and many complicated Problems by Professor Subhashish Chattopadhyay ” for IIT-JEE, I.Sc., CBSE, Karnataka PU, State Boards etc. CBSE Standard 12 and IIT-JEE Physics Survival Guide – CBSE & IIT-JEE Physics Survival Guide – SHM by Professor Subhashish Chattopadhyay SKMClasses Bangalore Useful for I.Sc. PU-II COMED-K CEE IGCSE IB AP-Chemistry, CET, VIT, Manipal, SRM and other exams.
This e-Book covers several examples of SHM Approximate Oscillations etc are also covered. There are many kinds of Problems which are NOT covered in Professor H C Verma ‘s books ( Concepts of Physics ) or Irodov, or ” Resnick & Halliday “. Some examples being split Lenses, Fresnel’s Biprism, Polytropic Processes, Silvered lenses, Slab with a lens like hole or filled with liquids, Cylindrical lenses, isodiaphers, Spallation Reaction, Magic Numbers, Doubly Magic Numbers, Metamaterials with Negative Refractive Index etc. All these kinds of Questions which have been asked in various exams are covered in eBooks of Professor Subhashish Chattopadhyay. Several Complicated examples and many more, and various incomplete dictionary kinds of collection for Course of IIT-JEE, CET, COMED-K etc with CBSE, CEE, IIT-JEE ( Main and Advanced ) Problems and Solutions. Includes NCERT / CBSE Text Book Solutions, Chapter wise Solutions, AIEEE ( Now known as IIT-JEE main ) Solutions, Roorkey Entrance Exam Solutions, EAMCET Solutions.
cbse-iit-jee-physics-survival-guide-shm-harmonic-oscillations-or-periodic-motion-by-prof-subhashish
:-{D
5 ] CBSE & IIT-JEE Physics Survival Guide – Sound Waves, Doppler Effect, Standing waves in Open Tube, Closed Tube, Rods or Bars by Professor Subhashish Chattopadhyay, Bangalore
Description – “ Spoon Feeding CBSE & IIT-JEE Physics Survival Guide – Sound Waves, Doppler Effect, Standing waves in Open Tube, Closed Tube, Rods or Bars by Professor Subhashish Chattopadhyay ” for IIT-JEE, I.Sc., CBSE, Karnataka PU, State Boards etc. CBSE Standard 12 and IIT-JEE Physics Survival Guide – CBSE & IIT-JEE Physics Survival Guide – Sound Waves, Oscillations in Wires by Professor Subhashish Chattopadhyay SKMClasses Bangalore Useful for I.Sc. PU-II COMED-K CEE IGCSE IB AP-Chemistry, CET, VIT, Manipal, SRM and other exams.
This e-Book covers several examples of Sound Waves, Doppler effect, Standing waves and Propagating Waves, Oscillations in Wires, Bars, Tubes ( both Open Tube and Closed Tube ) etc are also covered. There are many kinds of Problems which are NOT covered in Professor H C Verma ‘s books ( Concepts of Physics ) or Irodov, or ” Resnick & Halliday “. Some examples being split Lenses, Fresnel’s Biprism, Polytropic Processes, Silvered lenses, Slab with a lens like hole or filled with liquids, Cylindrical lenses, isodiaphers, Spallation Reaction, Magic Numbers, Doubly Magic Numbers, Metamaterials with Negative Refractive Index etc. All these kinds of Questions which have been asked in various exams are covered in eBooks of Professor Subhashish Chattopadhyay. Several Complicated examples and many more, and various incomplete dictionary kinds of collection for Course of IIT-JEE, CET, COMED-K etc with CBSE, CEE, IIT-JEE ( Main and Advanced ) Problems and Solutions. Includes NCERT / CBSE Text Book Solutions, Chapter wise Solutions, AIEEE ( Now known as IIT-JEE main ) Solutions, Roorkey Entrance Exam Solutions, EAMCET Solutions. Regarding the latest developments I wrote … “ GUT [ General Unified Theory ] is being modified to introduce a 5th fundamental force, because some heavy particles have been observed at CERN and various other experiments and Producing Gravitational waves at will, without mass, Madala Bosons to explain Dark Matter ”
cbse-iit-jee-physics-survival-guide-waves-and-sound-by-prof-subhashish
:-{D
4 ] CBSE & IIT-JEE Physics Survival Guide – Radio activity and Modern Physics by Professor Subhashish Chattopadhyay, Bangalore
Description – “ Spoon Feeding CBSE & IIT-JEE Physics Survival Guide – Radio activity and Modern Physics by Professor Subhashish Chattopadhyay ” for IIT-JEE, I.Sc., CBSE, Karnataka PU, State Boards etc. CBSE Standard 12 and IIT-JEE Physics Survival Guide – CBSE & IIT-JEE Physics Survival Guide – Radio activity and Modern Physics by Professor Subhashish Chattopadhyay SKMClasses Bangalore Useful for I.Sc. PU-II COMED-K CEE IGCSE IB AP-Chemistry, CET, VIT, Manipal, SRM and other exams.
This e-Book covers several examples of Radioactivity and Modern Physics. LASERS, Dirac Equation, Particle Physics, Diode, Triode, Transistor, Quantum Mechanics etc are also covered. There are many kinds of Problems which are NOT covered in Professor H C Verma ‘s books ( Concepts of Physics ) or Irodov, or ” Resnick & Halliday “. Some examples being split Lenses, Fresnel’s Biprism, Polytropic Processes, Silvered lenses, Slab with a lens like hole or filled with liquids, Cylindrical lenses, isodiaphers, Spallation Reaction, Magic Numbers, Doubly Magic Numbers, Metamaterials with Negative Refractive Index etc. All these kinds of Questions which have been asked in various exams are covered in eBooks of Professor Subhashish Chattopadhyay. Several Complicated examples and many more, and various incomplete dictionary kinds of collection for Course of IIT-JEE, CET, COMED-K etc with CBSE, CEE, IIT-JEE ( Main and Advanced ) Problems and Solutions. Includes NCERT / CBSE Text Book Solutions, Chapter wise Solutions, AIEEE ( Now known as IIT-JEE main ) Solutions, Roorkey Entrance Exam Solutions, EAMCET Solutions.
CBSE & IIT-JEE Physics Survival Guide-Radio activity and Modern Physics by Prof. Subhashish
:-{D
3 ] CBSE & IIT-JEE Physics Survival Guide – Mirrors Lenses Slabs Prisms Ray Diagram Problems – Optics by Professor Subhashish Chattopadhyay, Bangalore
Description – “ Spoon Feeding CBSE & IIT-JEE Physics Survival Guide – Mirrors Lenses Slabs Prisms Ray Diagram Problems Optics by Professor Subhashish Chattopadhyay ” for IIT-JEE, I.Sc., CBSE, Karnataka PU, State Boards etc. CBSE Standard 12 and IIT-JEE Physics Survival Guide – CBSE & IIT-JEE Physics Survival Guide – Mirrors Lenses Slabs Prisms Ray Diagram Problems Optics by Professor Subhashish Chattopadhyay SKMClasses Bangalore Useful for I.Sc. PU-II COMED-K CEE IGCSE IB AP-Chemistry, CET, VIT, Manipal, SRM and other exams.
This e-Book covers several examples of Mirror Slab Prism Lenses Ray Diagram Problems & Solutions Optics. Silvered Slab, Silvered Lenses, Silvered prisms are also covered. There are many kinds of Problems which are NOT covered in Professor H C Verma ‘s books ( Concepts of Physics ) or Irodov, or ” Resnick & Halliday “. Some examples being split Lenses, Fresnel’s Biprism, Polytropic Processes, Silvered lenses, Slab with a lens like hole or filled with liquids, Cylindrical lenses, isodiaphers, Spallation Reaction, Magic Numbers, Doubly Magic Numbers, Metamaterials with Negative Refractive Index etc. All these kinds of Questions which have been asked in various exams are covered in eBooks of Professor Subhashish Chattopadhyay. Several Complicated examples and many more, and various incomplete dictionary kinds of collection for Course of IIT-JEE, CET, COMED-K etc with CBSE, CEE, IIT-JEE ( Main and Advanced ) Problems and Solutions. Includes NCERT / CBSE Text Book Solutions, Chapter wise Solutions, AIEEE ( Now known as IIT-JEE main ) Solutions, Roorkey Entrance Exam Solutions, EAMCET Solutions.
In this eBook I wrote about my Personal Choice of, ” List of Best Experiments ” …
Michelson–Morley experiment proving there was no Aether, Moseley ‘s experiment with X-Rays to discover Protons, Jagadish chandra Bose demonstrating controlled emission / transmission and receiving of Radio waves, Casimir experiments to show Casimir forces of virtual particles, Eddington measuring bending of light, Flying atomic clocks in planes and confirming slowing down of time at high speeds, Victor Hess measured Radiation level variation at ground and high up in the atmosphere, Soviet physicist Sergey Vernov was the first to use radiosondes to perform cosmic ray readings with an instrument carried to high altitude by a balloon at heights up to 13.6 km, The proof of time dilation by Muon decay, Measurement of Space-time curvature near Earth and thereby the stress–energy tensor (which is related to the distribution and the motion of matter in space) in and near Earth , Detecting Gravitational Waves.
CBSE & IIT-JEE Physics Survival Guide-Mirrors Prisms Lens Slabs Optics by Prof. Subhashish
:-{D
2 ] CBSE & IIT-JEE Physics Survival Guide – Wave Optics by Professor Subhashish Chattopadhyay, Bangalore
Description – “ Spoon Feeding CBSE & IIT-JEE Physics Survival Guide – Wave Optics by Professor Subhashish Chattopadhyay ” for IIT-JEE, I.Sc., CBSE, Karnataka PU, State Boards etc. CBSE Standard 12 and IIT-JEE Physics Survival Guide – CBSE & IIT-JEE Physics Survival Guide- Wave Optics by Professor Subhashish Chattopadhyay SKMClasses Bangalore Useful for I.Sc. PU-II COMED-K CEE IGCSE IB AP-Chemistry, CET, VIT, Manipal, SRM and other exams.
This e-Book covers several examples of Wave Optics. Slabs, Silvered Slab, Lenses, Silvered Lenses, Prisms, Silvered prisms are also covered. There are many kinds of Problems which are NOT covered in Professor H C Verma ‘s books ( Concepts of Physics ) or Irodov, or ” Resnick & Halliday “. Some examples being split Lenses, Fresnel’s Biprism, Polytropic Processes, Silvered lenses, Slab with a lens like hole or filled with liquids, Cylindrical lenses, isodiaphers, Spallation Reaction, Magic Numbers, Doubly Magic Numbers, Metamaterials with Negative Refractive Index etc. All these kinds of Questions which have been asked in various exams are covered in eBooks of Professor Subhashish Chattopadhyay. Several Complicated examples and many more, and various incomplete dictionary kinds of collection for Course of IIT-JEE, CET, COMED-K etc with CBSE, CEE, IIT-JEE ( Main and Advanced ) Problems and Solutions. Includes NCERT / CBSE Text Book Solutions, Chapter wise Solutions, AIEEE ( Now known as IIT-JEE main ) Solutions, Roorkey Entrance Exam Solutions, EAMCET Solutions.
CBSE & IIT-JEE Physics Survival Guide-Wave Optics by Prof. Subhashish
:-{D
1 ] CBSE & IIT-JEE Physics Survival Guide – Ray or Geometrical Optics by Professor Subhashish Chattopadhyay, Bangalore
Description – “ Spoon Feeding CBSE & IIT-JEE Physics Survival Guide – Ray or Geometrical Optics by Professor Subhashish Chattopadhyay ” for IIT-JEE, I.Sc., CBSE, Karnataka PU, State Boards etc. CBSE Standard 12 and IIT-JEE Physics Survival Guide – CBSE & IIT-JEE Physics Survival Guide – Ray or Geometrical Optics by Professor Subhashish Chattopadhyay SKMClasses Bangalore Useful for I.Sc. PU-II COMED-K CEE IGCSE IB AP-Chemistry, CET, VIT, Manipal, SRM and other exams.
This e-Book covers several examples of Ray or Geometrical Optics. Slabs, Silvered Slab, Lenses, Silvered Lenses, Prisms, Silvered prisms are also covered. There are many kinds of Problems which are NOT covered in Professor H C Verma ‘s books ( Concepts of Physics ) or Irodov, or ” Resnick & Halliday “. Some examples being split Lenses, Fresnel’s Biprism, Polytropic Processes, Silvered lenses, Slab with a lens like hole or filled with liquids, Cylindrical lenses, isodiaphers, Spallation Reaction, Magic Numbers, Doubly Magic Numbers, Metamaterials with Negative Refractive Index etc. All these kinds of Questions which have been asked in various exams are covered in eBooks of Professor Subhashish Chattopadhyay. Several Complicated examples and many more, and various incomplete dictionary kinds of collection for Course of IIT-JEE, CET, COMED-K etc with CBSE, CEE, IIT-JEE ( Main and Advanced ) Problems and Solutions. Includes NCERT / CBSE Text Book Solutions, Chapter wise Solutions, AIEEE ( Now known as IIT-JEE main ) Solutions, Roorkey Entrance Exam Solutions, EAMCET Solutions.
CBSE & IIT-JEE Physics Survival Guide-Ray or Geometrical Optics by Prof. Subhashish
:-{D
Nima Arkani-Hamed has written a beautiful paper on ” The Future of Fundamental Physics “
We are too used to see ‘daily news ‘ which changes everyday. Often many of us start thinking or imagining Progress in Science and / or technology will also happen at that speed. Searching the net for future trends, every hour, actually wastes time, rather than teaching us anything. Slow long term prediction is difficult to do. These predictions does not change much. It needs very deep understanding of the present trends, to write about future.
:-{D
IIT-JEE, NCERT / CBSE, I.Sc., PU, Board exam, EAMCET, BITS Chemistry Books with lots of Examples ( Free pdf download of Chemistry Books, Chapter wise / Topic wise Questions and Solutions )
8 ] CBSE 12 & IIT-JEE Chemistry Survival Guide – Stoichiometry Titration by Prof. Subhashish Chattopadhyay
Description – “Spoon Feeding Stoichiometry Titration ” for IIT-JEE, I.Sc., CBSE, Karnataka PU, State Boards etc. CBSE Standard 12 and IIT-JEE Chemistry Survival Guide – Stoichiometry Titration by Prof. Subhashish Chattopadhyay SKMClasses Bangalore Useful for I.Sc. PU-II CEE IGCSE IB AP-Chemistry, CET, VIT, Manipal, SRM and other exams.
This e-Book covers several examples of Stoichiometry Titrations, Heating effects in several salts, colours or colors of the precipitates, Empirical formulae calculation, Limiting reagents, Titration examples, Equivalent weight, milli-equivalent weight, What mass or moles is reacting with how much ? How much is oxidised ? How much is Reduced ? Several Complicated examples and many more, and various incomplete dictionary kinds of collection for Course of IIT-JEE, CET, etc with CBSE, COMED-K, IIT-JEE ( Main and Advanced ) Problems and Solutions. Includes NCERT / CBSE Text Book Solutions, Chapter wise Solutions, AIEEE ( Now known as IIT-JEE main ) Solutions, Roorkey Entrance Exam Solutions, EAMCET Solutions.
CBSE 12 & IIT-JEE Chem Survival Guide-Stoichiometry Titration by Prof. Subhashish
:-{D
7 ] CBSE 12 & IIT-JEE Chemistry Survival Guide – Redox Reactions by Prof. Subhashish Chattopadhyay
Description – “Spoon Feeding Redox Reactions ” for IIT-JEE, I.Sc., CBSE, Karnataka PU, State Boards etc. CBSE Standard 12 and IIT-JEE Chemistry Survival Guide – Redox Reactions by Prof. Subhashish Chattopadhyay SKMClasses Bangalore Useful for I.Sc. PU-II CEE COMED-K IGCSE IB AP-Chemistry, CET, VIT, Manipal, SRM and other exams.
This e-Book covers more than 60 examples of Redox Reactions, Several Complicated examples and many more, and various incomplete dictionary kinds of collection for Course of IIT-JEE, CET, etc with CBSE, COMED-K, IIT-JEE ( Main and Advanced ) Problems and Solutions. Includes NCERT / CBSE Text Book Solutions, Chapter wise Solutions, AIEEE ( Now known as IIT-JEE main ) Solutions, Roorkey Entrance Exam Solutions, EAMCET Solutions.
CBSE 12 & IIT-JEE Chem Survival Guide-Redox Reactions by Prof. Subhashish
:-{D
6 ] CBSE 12 & IIT-JEE Chemistry Survival Guide – Electrochemistry by Prof. Subhashish Chattopadhyay
Description – “Spoon Feeding Electrochemistry ” for IIT-JEE, I.Sc., CBSE, Karnataka PU, State Boards etc. CBSE Standard 12 and IIT-JEE Chemistry Survival Guide – Electrochemistry by Prof. Subhashish Chattopadhyay SKMClasses Bangalore Useful for I.Sc. PU-II CEE IGCSE IB AP-Chemistry, CET, VIT, Manipal, SRM and other exams.
This e-Book covers Electrochemistry, Oxidation Potential, Reduction Potential, Electrode Potential, Reactivity Series, Battery, Nernst Equation, Variation of Voltage with concentration, Electrolyte, Electrolysis, Salt Bridge, Daniel Cell, Primary Cell, Secondary Cell, Galvanic Cell, Electrolytic Cell, Conductivity, Kohlrausch ’s Law and many more, and various incomplete dictionary kinds of collection for Course of IIT-JEE, CET, etc with CBSE, COMED-K, IIT-JEE ( Main and Advanced ) Problems and Solutions. Includes NCERT / CBSE Text Book Solutions, Chapter wise Solutions, AIEEE ( Now known as IIT-JEE main ) Solutions, Roorkey Entrance Exam Solutions, EAMCET Solutions.
CBSE 12 & IIT-JEE Chem Survival Guide-ElectroChemistry by Prof. Subhashish
:-{D
5 ] CBSE 12 & IIT-JEE Organic Chemistry Survival Guide – Reduction Methods by Prof. Subhashish Chattopadhyay
Description – “Spoon Feeding Reduction Methods ” for IIT-JEE, I.Sc., CBSE, Karnataka PU, State Boards etc. CBSE Standard 12 and IIT-JEE Organic Chemistry Survival Guide – Reduction Methods by Prof. Subhashish Chattopadhyay SKMClasses Bangalore Useful for I.Sc. PU-II CEE COMED-K IGCSE IB AP-Chemistry, CET, VIT, Manipal, SRM and other exams.
This e-Book covers Various kinds of Reduction Methods in Organic Chemistry. Covers Gilmann ’s Reagent, Grignard Reagent, Trimethyl Silyl Iodide, Silyl Wittig Reaction, Hydrogen with Ni, Zn, Pd Palladium, Bakers Yeast, Wolf Kishner, Wilkinson ’s Catalyst, Birch Reduction, Lindlar ’s Catalyst, Benkeser Reduction, Reduction with HCO2H, Sodium Boro Hydride NaBH4, Veils Meier Reaction, Luche ’s Reagent, Super Hydride, Sodium Cyno boro hydride, Dibal H, Adams Catalyst, Rosen Mund Reduction, Various Lithium Aluminium Hydrides, NaNH2, and many more, and various incomplete dictionary kinds of collection for Course of IIT-JEE, CET, etc with CBSE, COMED-K, IIT-JEE ( Main and Advanced ) Problems and Solutions. Includes NCERT / CBSE Text Book Solutions, Chapter wise Solutions, AIEEE ( Now known as IIT-JEE main ) Solutions, Roorkey Entrance Exam Solutions, EAMCET Solutions.
CBSE 12 & IIT-JEE Organic Chem Survival Guide-Reduction methods by Prof. Subhashish
:-{D
4 ] CBSE 12 & IIT-JEE Organic Chemistry Survival Guide – Oxidation Methods by Prof. Subhashish Chattopadhyay
Description – “Spoon Feeding Oxidation Methods ” for IIT-JEE, I.Sc., CBSE, Karnataka PU, State Boards etc. CBSE Standard 12 and IIT-JEE Organic Chemistry Survival Guide – Oxidation Methods by Prof. Subhashish Chattopadhyay SKMClasses Bangalore Useful for I.Sc. PU-II CEE COMED-K IGCSE IB AP-Chemistry, CET, VIT, Manipal, SRM and other exams.
This e-Book covers Various kinds of Oxidation Methods in Organic Chemistry. Covers Sarett ’s Reagent, PCC, Chromium Oxide, Osmium Oxide, Manganese Oxide, Silver oxides, Ruthenium Oxide, Hydrogen Peroxide, Selenium dioxide, KMnO4, Jones, Julia Colonna, DCC, Corey ’s, Moffats, Ley Oxidation, MPV, Fetizon, Fremy ’s Salt, Elbs Persulphate Oxidation, Sodiumperiodate, Palladium Chloride, Copper Chloride, Sharpless epoxidation, and many more, and various incomplete dictionary kinds of collection for Course of IIT-JEE, CET, etc with CBSE, COMED-K, IIT-JEE ( Main and Advanced ) Problems and Solutions.Includes NCERT / CBSE Text Book Solutions, Chapter wise Solutions, AIEEE ( Now known as IIT-JEE main ) Solutions, Roorkey Entrance Exam Solutions, EAMCET Solutions.
CBSE 12 & IIT-JEE Organic Chem Survival Guide-Oxidation methods by Prof. Subhashish
:-{D
3 ] CBSE 12 & IIT-JEE Chem Survival Guide – Bonds & Structure by Prof. Subhashish Chattopadhyay
Description – “Spoon Feeding Bonds & Structures ” for IIT-JEE, I.Sc., CBSE, Karnataka PU, State Boards etc. CBSE Standard 12 and IIT-JEE Chem Survival Guide – Bonds & Structures by Prof. Subhashish Chattopadhyay SKMClasses Bangalore Useful for I.Sc. PU-II CEE COMED-K IGCSE IB AP-Chemistry, CET, VIT, Manipal, SRM and other exams.
This e-Book covers Various kinds of Bonds and Structures in Chemistry. Covers Sigma, Pi, Delta, Back Bonding, Coordinate or Dative Bond, Eta Bond, Hydrogen Bond, London forces, and many more, and various incomplete dictionary kinds of collection for Course of IIT-JEE, CET, etc with CBSE, COMED-K, IIT-JEE ( Main and Advanced ) Problems and Solutions.Includes NCERT / CBSE Text Book Solutions, Chapter wise Solutions, AIEEE ( Now known as IIT-JEE main ) Solutions, Roorkey Entrance Exam Solutions, EAMCET Solutions.
CBSE 12 & IIT-JEE Chem Survival Guide-Bonds & Structure by Prof. Subhashish
:-{D
2 ] CBSE 12 & IIT-JEE Chem Survival Guide – Elements & Properties by Prof. Subhashish Chattopadhyay
Description – “Spoon Feeding Elements & Properties ” for IIT-JEE, I.Sc., CBSE, Karnataka PU, State Boards etc. CBSE Standard 12 and IIT-JEE Chem Survival Guide – Elements & Properties by Prof. Subhashish Chattopadhyay SKMClasses Bangalore Useful for I.Sc. PU-II CEE COMED-K IGCSE IB AP-Chemistry, CET, VIT, Manipal, SRM and other exams.
This e-Book covers Elements & Their Properties in Chemistry. Covers the discoveries by spectral Analysis, Named after smell, places, people etc. Various compounds, tests, properties, and various incomplete dictionary kinds of collection for Course of IIT-JEE, CET, etc with CBSE, COMED-K, IIT-JEE ( Main and Advanced ) Problems and Solutions.Includes NCERT / CBSE Text Book Solutions, Chapter wise Solutions, AIEEE ( Now known as IIT-JEE main ) Solutions, Roorkey Entrance Exam Solutions, EAMCET Solutions.
CBSE 12 & IIT-JEE Chem Survival Guide-Elements & Properties by Prof. Subhashish
:-{D
1 ] CBSE 12 & IIT-JEE Chem Survival Guide – Empirical Formulae by Prof. Subhashish Chattopadhyay
Description – “Spoon Feeding Empirical Formulae ” for IIT-JEE, I.Sc., CBSE, Karnataka PU, State Boards etc. CBSE Standard 12 and IIT-JEE Chem Survival Guide – Empirical Formulae by Prof. Subhashish Chattopadhyay SKMClasses Bangalore Useful for I.Sc. PU-II CEE COMED-K IGCSE IB AP-Chemistry, CET, VIT, Manipal, SRM and other exams.
This e-Book covers various kinds of Empirical Equations in Chemistry. These equations are formed by experiments, and graph plotting. In some rare cases the Theory was developed later. Covers Slater’s rule, Shielding, Finding Electronegativity values by Allred and Rochow ’s empirical formula, Moseley’s Law, Trouton ’s law, Einstein-Debey equation (Dulong & Petit), Reynolds number, Raoult’s law, Variation of viscosity with temperature, Arrhenius model, Williams-Landel-Ferry model, Masuko and Magill model, Walther formula, Wright model, Seeton model, Variation of surface tension with temperature, Eotvos equation, Guggenheim-Katayama equation, Debye-Huckel-Onsager theory of conductivity of ions in dilute solutions, Liquid drop model of Nucleus, Nuclear Shell Model, Ionic character percentage of a diatomic molecule, and various incomplete dictionary kinds of collection for Course of IIT-JEE, CET, etc with CBSE, COMED-K, IIT-JEE ( Main and Advanced ) Problems and Solutions.Includes NCERT / CBSE Text Book Solutions, Chapter wise Solutions, AIEEE ( Now known as IIT-JEE main ) Solutions, Roorkey Entrance Exam Solutions, EAMCET Solutions.
CBSE 12 & IIT-JEE Chem Survival Guide-Empirical Formulae by Prof. Subhashish
:-{D
IIT-JEE, NCERT / CBSE, I.Sc., PU, Board exam, EAMCET, BITS Math Books with lots of Questions and Solutions, Examples ( Free pdf download of Math Books, Chapter wise / Topic wise Solutions )
17 ] CBSE & IIT-JEE Math Survival Guide – Trigonometry by Prof. Subhashish Chattopadhyay
Description – “Spoon Feeding Trigonometry” for IIT-JEE, I.Sc., CBSE, Karnataka PU, State Boards etc. CBSE Standard 12 Math Survival Guide – Trigonometry by Prof. Subhashish Chattopadhyay SKMClasses Bangalore. Useful for I.Sc. PU-II CET CEE COMED-K IGCSE IB AP-Mathematics and other exams.
This e-Book covers Trigonometry with lots of Video explanations. The classroom teaching videos can be seen by clicking on the given links. The videos can be downloaded also. Hundreds of tricky problems solved. Rules / Tricks / Properties of Trigonometry, with CBSE, COMED-K, IIT-JEE ( Main and Advanced ) Problems and Solutions. Includes NCERT / CBSE Text Book Solutions, Chapter wise Solutions, AIEEE ( Now known as IIT-JEE main ) Solutions, Roorkey Entrance Exam Solutions, EAMCET Solutions. R D Sharma Solutions, R S Aggarwal’s Solutions.
CBSE & IIT-JEE Math Survival Guide-Trigonometry by Prof. Subhashish
😀
16 ] CBSE & IIT-JEE Math Survival Guide – 3D Coordinate Geometry by Prof. Subhashish Chattopadhyay
Description – “Spoon Feeding 3D Coordinate Geometry” for IIT-JEE, I.Sc., CBSE, Karnataka PU, State Boards etc. CBSE Standard 12 Math Survival Guide – 3D Coordinate Geometry by Prof. Subhashish Chattopadhyay SKMClasses Bangalore. Useful for I.Sc. PU-II CET CEE COMED-K IGCSE IB AP-Mathematics and other exams.
This e-Book covers 3D Coordinate Geometry with lots of Video explanations. The classroom teaching videos can be seen by clicking on the given links. The videos can be downloaded also. Hundreds of tricky problems solved. Rules / Tricks / Properties of 3D Coordinate Geometry, with CBSE, COMED-K, IIT-JEE ( Main and Advanced ) Problems and Solutions. Includes NCERT / CBSE Text Book Solutions, Chapter wise Solutions, AIEEE ( Now known as IIT-JEE main ) Solutions, Roorkey Entrance Exam Solutions, EAMCET Solutions. R D Sharma Solutions, R S Aggarwal’s Solutions.
CBSE & IIT-JEE Math Survival Guide-3D Geometry by Prof. Subhashish
:-{D
15 ] CBSE & IIT-JEE Math Survival Guide – Hyperbola Coordinate Geometry by Prof. Subhashish Chattopadhyay
Description – “Spoon Feeding Hyperbola Coordinate Geometry” for IIT-JEE, I.Sc., CBSE, Karnataka PU, State Boards etc. CBSE Standard 12 Math Survival Guide – Hyperbola Coordinate Geometry by Prof. Subhashish Chattopadhyay SKMClasses Bangalore. Useful for I.Sc. PU-II CET CEE COMED-K IGCSE IB AP-Mathematics and other exams.
This e-Book covers Hyperbola Coordinate Geometry with lots of Video explanations. The classroom teaching videos can be seen by clicking on the given links. The videos can be downloaded also. Hundreds of tricky problems solved. Rules / Tricks / Properties of Hyperbola Coordinate Geometry, with CBSE, COMED-K, IIT-JEE ( Main and Advanced ) Problems and Solutions. Includes NCERT / CBSE Text Book Solutions, Chapter wise Solutions, AIEEE ( Now known as IIT-JEE main ) Solutions, Roorkey Entrance Exam Solutions, EAMCET Solutions. R D Sharma Solutions, R S Aggarwal’s Solutions.
CBSE & IIT-JEE Math Survival Guide-Hyperbola by Prof. Subhashish
:-{D
14 ] CBSE & IIT-JEE Math Survival Guide – Ellipse Coordinate Geometry by Prof. Subhashish Chattopadhyay
Description – “Spoon Feeding Ellipse Coordinate Geometry” for IIT-JEE, I.Sc., CBSE, Karnataka PU, State Boards etc. CBSE Standard 12 Math Survival Guide – Ellipse Coordinate Geometry by Prof. Subhashish Chattopadhyay SKMClasses Bangalore. Useful for I.Sc. PU-II CET CEE COMED-K IGCSE IB AP-Mathematics and other exams.
This e-Book covers Ellipse Coordinate Geometry with lots of Video explanations. The classroom teaching videos can be seen by clicking on the given links. The videos can be downloaded also. Hundreds of tricky problems solved. Rules / Tricks / Properties of Ellipse Coordinate Geometry, with CBSE, COMED-K, IIT-JEE ( Main and Advanced ) Problems and Solutions. Includes NCERT / CBSE Text Book Solutions, Chapter wise Solutions, AIEEE ( Now known as IIT-JEE main ) Solutions, Roorkey Entrance Exam Solutions, EAMCET Solutions. R D Sharma Solutions, R S Aggarwal’s Solutions.
CBSE & IIT-JEE Math Survival Guide-Ellipse by Prof. Subhashish
:-{D
13 ] CBSE & IIT-JEE Math Survival Guide – Parabola Coordinate Geometry by Prof. Subhashish Chattopadhyay
Description – “Spoon Feeding Parabola Coordinate Geometry” for IIT-JEE, I.Sc., CBSE, Karnataka PU, State Boards etc. CBSE Standard 12 Math Survival Guide – Parabola Coordinate Geometry by Prof. Subhashish Chattopadhyay SKMClasses Bangalore. Useful for I.Sc. PU-II CET CEE COMED-K IGCSE IB AP-Mathematics and other exams.
This e-Book covers Parabola Coordinate Geometry with lots of Video explanations. The classroom teaching videos can be seen by clicking on the given links. The videos can be downloaded also. Hundreds of tricky problems solved. Rules / Tricks / Properties of Parabola Coordinate Geometry, with CBSE, COMED-K, IIT-JEE ( Main and Advanced ) Problems and Solutions. Includes NCERT / CBSE Text Book Solutions, Chapter wise Solutions, AIEEE ( Now known as IIT-JEE main ) Solutions, Roorkey Entrance Exam Solutions, EAMCET Solutions. R D Sharma Solutions, R S Aggarwal ’s Solutions.
CBSE & IIT-JEE Math Survival Guide-Parabola by Prof. Subhashish
:-{D
12 ] CBSE & IIT-JEE Math Survival Guide – Pair of Straight Lines Coordinate Geometry by Prof. Subhashish Chattopadhyay
Description – “Spoon Feeding Pair of Straight Lines Coordinate Geometry” for IIT-JEE, I.Sc., CBSE, Karnataka PU, State Boards etc. CBSE Standard 12 Math Survival Guide – Pair of Straight Lines Coordinate Geometry by Prof. Subhashish Chattopadhyay SKMClasses Bangalore. Useful for I.Sc. PU-II CET CEE COMED-K IGCSE IB AP-Mathematics and other exams.
This e-Book covers Pair of Straight Lines Coordinate Geometry with lots of Video explanations. The classroom teaching videos can be seen by clicking on the given links. The videos can be downloaded also. Hundreds of tricky problems solved. Rules / Tricks / Properties of Pair of Straight Lines Coordinate Geometry, with CBSE, COMED-K, IIT-JEE ( Main and Advanced ) Problems and Solutions. Includes NCERT / CBSE Text Book Solutions, Chapter wise Solutions, AIEEE ( Now known as IIT-JEE main ) Solutions, Roorkey Entrance Exam Solutions, EAMCET Solutions. R D Sharma Solutions, R S Aggarwal’s Solutions.
CBSE & IIT-JEE Math Survival Guide-Pair of Straight Lines by Prof. Subhashish
:-{D
11 ] CBSE 11 & IIT-JEE Math Survival Guide – Circles Coordinate Geometry by Prof. Subhashish Chattopadhyay
Description – “Spoon Feeding Circles Coordinate Geometry” for IIT-JEE, I.Sc. , CBSE, Karnataka PU, State Boards etc. CBSE Standard 12 Math Survival Guide – Circles Coordinate Geometry by Prof. Subhashish Chattopadhyay SKMClasses Bangalore. Useful for I.Sc. PU-II CET CEE COMED-K IGCSE IB AP-Mathematics and other exams.
This e-Book covers Circles Coordinate Geometry with lots of Video explanations. The classroom teaching videos can be seen by clicking on the given links. The videos can be downloaded also. Hundreds of tricky problems solved. Rules / Tricks / Properties of Circles Coordinate Geometry, with CBSE, COMED-K, IIT-JEE ( Main and Advanced ) Problems and Solutions. Includes NCERT / CBSE Text Book Solutions, Chapter wise Solutions, AIEEE ( Now known as IIT-JEE main ) Solutions, Roorkey Entrance Exam Solutions, EAMCET Solutions. R D Sharma Solutions, R S Aggarwal’s Solutions.
CBSE 11 & IIT-JEE Math Survival Guide-Circles by Prof. Subhashish
:-{D
10 ] CBSE 11 & IIT-JEE Math Survival Guide – Straight Lines Coordinate Geometry by Prof. Subhashish Chattopadhyay
Description – “Spoon Feeding Lines Coordinate Geometry” for IIT-JEE, I.Sc. , CBSE, Karnataka PU, State Boards etc. CBSE Standard 12 Math Survival Guide – Lines Coordinate Geometry by Prof. Subhashish Chattopadhyay SKMClasses Bangalore. Useful for I.Sc. PU-II CET CEE COMED-K IGCSE IB AP-Mathematics and other exams.
This e-Book covers Straight Lines Coordinate Geometry with lots of Video explanations. The classroom teaching videos can be seen by clicking on the given links. The videos can be downloaded also. Hundreds of tricky problems solved. Rules / Tricks / Properties of Straight Lines Coordinate Geometry, with CBSE, COMED-K, IIT-JEE ( Main and Advanced ) Problems and Solutions. Includes NCERT / CBSE Text Book Solutions, Chapter wise Solutions, AIEEE ( Now known as IIT-JEE main ) Solutions, Roorkey Entrance Exam Solutions, EAMCET Solutions. R D Sharma Solutions, R S Aggarwal’s Solutions.
CBSE 11 & IIT-JEE Math Survival Guide-Straight Lines by Prof. Subhashish
:-{D
9 ] CBSE 11 & IIT-JEE Math Survival Guide – Complex Numbers or Imaginary Numbers by Prof. Subhashish Chattopadhyay
Description – “Spoon Feeding Complex Numbers or Imaginary Numbers” for IIT-JEE, I.Sc. , CBSE, Karnataka PU, State Boards etc. CBSE Standard 12 Math Survival Guide – Complex Numbers or Imaginary Numbers by Prof. Subhashish Chattopadhyay SKMClasses Bangalore. Useful for I.Sc. PU-II CET CEE COMED-K IGCSE IB AP-Mathematics and other exams.
This e-Book covers Complex Numbers or Imaginary Numbers with lots of Video explanations. The classroom teaching videos can be seen by clicking on the given links. The videos can be downloaded also. Hundreds of tricky problems solved. Rules / Tricks / Properties of Complex Numbers or Imaginary Numbers, with CBSE, COMED-K, IIT-JEE ( Main and Advanced ) Problems and Solutions. Includes NCERT / CBSE Text Book Solutions, Chapter wise Solutions, AIEEE ( Now known as IIT-JEE main ) Solutions, Roorkey Entrance Exam Solutions, EAMCET Solutions. R D Sharma Solutions, R S Aggarwal ’s Solutions.
CBSE 11 & IIT-JEE Math Survival Guide-Complex Number by Prof. Subhashish
:-{D
8 ] CBSE 12 & IIT-JEE Math Survival Guide – Quadratic Equations by Prof. Subhashish Chattopadhyay
Description – “Spoon Feeding Quadratic Equations” for IIT-JEE, I.Sc. , CBSE, Karnataka PU, State Boards etc. CBSE Standard 12 Math Survival Guide – Quadratic Equation by Prof. Subhashish Chattopadhyay SKMClasses Bangalore. Useful for I.Sc. PU-II CET CEE COMED-K IGCSE IB AP-Mathematics and other exams.
This e-Book covers Quadratic Equations with lots of Video explanations. The classroom teaching videos can be seen by clicking on the given links. The videos can be downloaded also. Hundreds of tricky problems solved. Rules / Tricks / Properties of Quadratic Equations, with CBSE, COMED-K, IIT-JEE ( Main and Advanced ) Problems and Solutions. Includes NCERT / CBSE Text Book Solutions, Chapter wise Solutions, AIEEE ( Now known as IIT-JEE main ) Solutions, Roorkey Entrance Exam Solutions, EAMCET Solutions. R D Sharma Solutions, R S Aggarwal’s Solutions.
CBSE 11 & IIT-JEE Math Survival Guide-Quadratic Equation by Prof. Subhashish
:-{D
7 ] CBSE 12 & IIT-JEE Math Survival Guide – Continuity and Differentiability by Prof. Subhashish Chattopadhyay
Description – “Spoon Feeding Continuity & Differentiability” for IIT-JEE, I.Sc. , CBSE, Karnataka PU, State Boards etc. CBSE Standard 12 Math Survival Guide – Continuity and Differentiability by Prof. Subhashish Chattopadhyay SKMClasses Bangalore. Useful for I.Sc. PU-II CET CEE COMED-K IGCSE IB AP-Mathematics and other exams.
This e-Book covers Continuity and Differentiability with lots of Video explanations. The classroom teaching videos can be seen by clicking on the given links. The videos can be downloaded also. Hundreds of tricky problems solved. Rules / Tricks / Properties of Continuity and Differentiability, with CBSE, COMED-K, IIT-JEE ( Main and Advanced ) Problems and Solutions. Includes NCERT / CBSE Text Book Solutions, Chapter wise Solutions, AIEEE ( Now known as IIT-JEE main ) Solutions, Roorkey Entrance Exam Solutions, EAMCET Solutions. R D Sharma Solutions, R S Aggarwal ’s Solutions.
CBSE 12 & IIT-JEE Math Survival Guide-Continuity & Differentiability by Prof. Subhashish
:-{D
6 ] CBSE 12 & IIT-JEE Math Survival Guide – Relations and Functions by Prof. Subhashish Chattopadhyay
Description – “Spoon Feeding Relations & Functions” for IIT-JEE, I.Sc. , CBSE, Karnataka PU, State Boards etc. CBSE Standard 12 Math Survival Guide – Relations and Functions by Prof. Subhashish Chattopadhyay SKMClasses Bangalore Useful for I.Sc. PU-II CET CEE COMED-K IGCSE IB AP-Mathematics and other exams.
This e-Book covers Relations and Functions with lots of Video explanations. The classroom teaching videos can be seen by clicking on the given links. The videos can be downloaded also. Hundreds of tricky problems solved. Rules / Tricks / Properties of Relations and Functions, with CBSE, COMED-K, IIT-JEE ( Main and Advanced ) Problems and Solutions. Includes NCERT / CBSE Text Book Solutions, Chapter wise Solutions, AIEEE ( Now known as IIT-JEE main ) Solutions, Roorkey Entrance Exam Solutions, EAMCET Solutions. R D Sharma Solutions, R S Aggarwal ’s Solutions.
CBSE 12 & IIT-JEE Math Survival Guide-Relations & Functions by Prof. Subhashish
:-{D
5 ] CBSE 12 & IIT-JEE Math Survival Guide – Graphs and Functions by Prof. Subhashish Chattopadhyay
Description – “Spoon Feeding Graphs & Functions” for IIT-JEE, I.Sc. , CBSE, Karnataka PU, State Boards etc. CBSE Standard 12 Math Survival Guide – Graphs and Functions by Prof. Subhashish Chattopadhyay SKMClasses Bangalore Useful for I.Sc. PU-II CET CEE COMED-K IGCSE IB AP-Mathematics and other exams.
This e-Book covers Graphs and Functions with lots of Video explanations. The classroom teaching videos can be seen by clicking on the given links. The videos can be downloaded also. Hundreds of tricky problems solved. Rules / Tricks / Properties of Graphs and Functions, with CBSE, COMED-K, IIT-JEE ( Main and Advanced ) Problems and Solutions. Includes NCERT / CBSE Text Book Solutions, Chapter wise Solutions, AIEEE ( Now known as IIT-JEE main ) Solutions, Roorkey Entrance Exam Solutions, EAMCET Solutions. R D Sharma Solutions, R S Aggarwal’s Solutions.
CBSE 12 & IIT-JEE Math Survival Guide-Functions & Graphs by Prof. Subhashish
:-{D
4 ] CBSE 12 & IIT-JEE Math Survival Guide – Indefinite Integrals by Prof. Subhashish Chattopadhyay
Description – “Spoon Feeding Indefinite Integrals & Calculus” for IIT-JEE, I.Sc. , CBSE, Karnataka PU, State Boards etc. CBSE Standard 12 Math Survival Guide – Indefinite Integrals by Prof. Subhashish Chattopadhyay SKMClasses Bangalore Useful for I.Sc. PU-II CET CEE IGCSE IB AP-Mathematics and other exams.
This e-Book covers Indefinite Integrals with lots of Video explanations. The classroom teaching videos can be seen by clicking on the given links. The videos can be downloaded also. Hundreds of tricky problems solved. Rules / Tricks / Properties of Indefinite Integrals, with CBSE, COMED-K, IIT-JEE ( Main and Advanced ) Problems and Solutions. Includes NCERT / CBSE Text Book Solutions, Chapter wise Solutions, AIEEE ( Now known as IIT-JEE main ) Solutions, Roorkey Entrance Exam Solutions, EAMCET Solutions. R D Sharma Solutions, R S Aggarwal’s Solutions.
CBSE 12 & IIT-JEE Math Survival Guide-Indefinite Integrals by Prof. Subhashish
:-{D
3 ] CBSE 12 & IIT-JEE Math Survival Guide – Area & Volume by Prof. Subhashish Chattopadhyay
Description – “Spoon Feeding Area and Volume ” for IIT-JEE, I.Sc. , CBSE, Karnataka PU, State Boards etc. CBSE Standard 12 and IIT-JEE Math Survival Guide – Area and Volume by Prof. Subhashish Chattopadhyay SKMClasses Bangalore Useful for I.Sc. PU-II CET CEE IGCSE IB AP-Mathematics and other exams.
This e-Book covers various kinds of graphs, such as graph of Ln x, ( ln x )/x, x Ln x, floor x [ x ] , Shifting of graphs, roots of Quadratic, cubic, and other higher powers of x ( polynomials ), asymptotes, ( How to find Asymptotes ) etc. Volume by revolution and hundreds of Area problems of IIT-JEE, CET, etc with CBSE, COMED-K, IIT-JEE ( Main and Advanced ) Problems and Solutions.Includes NCERT / CBSE Text Book Solutions, Chapter wise Solutions, AIEEE ( Now known as IIT-JEE main ) Solutions, Roorkey Entrance Exam Solutions, EAMCET Solutions. R D Sharma Solutions, R S Aggarwal ’s Solutions.
CBSE 12 & IIT-JEE Math Survival Guide-Area & Volume by Prof. Subhashish
:-{D
2 ] CBSE 12 & IIT-JEE Math Survival Guide – Definite Integrals by Prof. Subhashish Chattopadhyay
Description – “Spoon Feeding Definite Integrals ” for IIT-JEE, I.Sc. , CBSE, Karnataka PU, State Boards etc. CBSE Standard 12 Math Survival Guide-Definite Integrals by Prof. Subhashish Chattopadhyay SKMClasses Bangalore Useful for I.Sc. PU-II CET CEE IGCSE IB AP-Mathematics and other exams.
CBSE 12 & IIT-JEE Math Survival Guide-Definite Integrals by Prof. Subhashish
This e-Book covers Definite Integrals with [ x ] greatest integer functions, { x } fraction function, Max and Min functions. Gamma function, Beta function, Integration after converting to Complex number, Leibnitz forms of Differentiating Integrals, L Hospital’s rule applied to limits with Integrals, Inequalities of Integrals, Rules / Tricks / Properties of Definite Integrals, with CBSE, COMED-K, IIT-JEE ( Main and Advanced ) Problems and Solutions.Includes NCERT / CBSE Text Book Solutions, Chapter wise Solutions, AIEEE ( Now known as IIT-JEE main ) Solutions, Roorkey Entrance Exam Solutions, EAMCET Solutions. R D Sharma Solutions, R S Aggarwal’s Solutions.
:-{D
1 ] CBSE 12 Math Survival Guide – Differential Equations by Prof. Subhashish Chattopadhyay
Description – “Spoon Feeding Differential Equations ” for IIT-JEE, I.Sc. , CBSE, Karnataka PU, State Boards etc. CBSE Standard 12 Math Survival Guide – Differential Equations by Prof. Subhashish Chattopadhyay SKMClasses Bangalore Useful for I.Sc. PU-II CET CEE IGCSE IB AP-Mathematics and other exams.
CBSE 12 & IIT-JEE Math Survival Guide-Differential Equations by Prof. Subhashish
This e-Book covers all kinds of Differential equations, and methods to solve them. There is a priority checklist for the approach to be taken for solving the problems. Covers ISc, CBSE, COMED-K IIT-JEE problems, Linear, Homogeneous, Variable separable by substitution, Exact, Reducible to exact, Bernoulli, Integrating Factors or Multiplying Factors, even Clairaut’s Differential Equations ( IIT-JEE 1999, Bihar CEE 1999 ). Includes NCERT / CBSE Text Book Solutions, Chapter wise Solutions, AIEEE ( Now known as IIT-JEE main ) Solutions, Roorkey Entrance Exam Solutions, EAMCET Solutions. R D Sharma Solutions, R S Aggarwal ’s Solutions.
:-{D
Various States have different names for the Engineering Entrance Exams.
CET – Common Engineering Entrance Test or Common Entrance Test is for Karnataka, Maharastra, Gujrat, Himachal Pradesh, J&K
GUJCET Exam – Gujarat Entrance Common Entrance Test – Engineering
HPCET – Himachal Pradesh Common Entrance Test
CEE – Commissionerate of Entrance Examinations Kerala. Some people say Common Entrance Exam. The exam in Kerala actually is known as KEAM – Kerala
Engineering Agriculture Medical Degree.
ASSAM CEE – Assam Combined Entrance Exam
EAMCET – Engineering and Medical Common Entrance Test
MP PET – Madhya Pradesh Pre Engineering Test. Randomly I liked lots of Physics Questions of MP-PET, as these were of very high quality / interesting.
RPET or R-PET – Rajasthan Pre Engineering Test
WBJEE or WB-JEE – West Bengal Joint Entrance Exam. The questions of these are very good / high quality.
UPSEE – Utter Pradesh State Entrance Exam
BCECEB – Bihar Combined Entrance Competitive Examination Board. The exam name is BCECE. Some call it as Bihar Combined Engineering Entrance Exam BCEEE or
BCECE (Bihar Combined Entrance Competitive Examination)
OJEE – Orissa Joint Entrance Exam
Tamilnadu does not have any state ( common ) entrance test. The admissions in colleges / universities are through standard 12 marks.
TNEA is a State Engineering Entrance Examination, which is conducted by Anna University. Tamil Nadu Engineering Admission.
COMEDK PGET – Consortium of Medical, Engineering and Dental Colleges of Karnataka for PG Post Graduate
NATA – National Aptitude Test in Architecture. National Institute of Advanced Studies in Architecture (NIASA) conducts this.
ISAT by IISAT – Indian Institute of Space Science and Technology (IISAT) Admission Test (ISAT) is a National Level Entrance Examination.
NAT – National Aptitude Test by Society for Research & Development in Education (SRDE), New Delhi
ENAT – EPSI National Admission Test. by Manipal Institute of Technology. Manipal Online Entrance Test Manipal-OET
VITEEE – VIT Engineering Entrance Exam, Vellore Institute of Technology. Conducted by VIT university
BITSAT – Birla Institute of Technology and Science Admission Test.
Punjab PET – Punjab Engineering Admission, Pre Engineering Test
ASSAM CEE – Assam Combined Entrance Exam
Tripura JEE – Tripura Joint Entrance Exam
NEE – NERIST Entrance Examination. Conducted by the North Eastern Regional Institute of Science & Technology (NERIST), Nirjuli, Itanagar, Arunachal Pradesh
1 ] CET CEE EAMCET JEE Math Survival Guide – Hyperbola Coordinate Geometry by Prof. Subhashish Chattopadhyay
Description – “Spoon Feeding Hyperbola Coordinate Geometry” for IIT-JEE, I.Sc., CBSE, Karnataka PU, State Boards etc. CBSE Standard 12 Math Survival Guide-Hyperbola Coordinate Geometry by Prof. Subhashish Chattopadhyay SKMClasses Bangalore. Useful for I.Sc. PU-II CET CEE COMED-K IGCSE IB AP-Mathematics and other exams.
This e-Book covers Hyperbola Coordinate Geometry with lots of Video explanations. The classroom teaching videos can be seen by clicking on the given links. The videos can be downloaded also. Hundreds of tricky problems solved. Rules / Tricks / Properties of Hyperbola Coordinate Geometry, with CBSE, COMED-K, IIT-JEE ( Main and Advanced ) Problems and Solutions. Includes NCERT / CBSE Text Book Solutions, Chapter wise Solutions, AIEEE ( Now known as IIT-JEE main ) Solutions, Roorkey Entrance Exam Solutions, CET, CEE, PET, EAMCET Solutions. R D Sharma Solutions, R S Aggarwal ’s Solutions.
CET CEE PET EAMCET JEE Math Survival Guide-Hyperbola by Prof. Subhashish
:-{D
https://zookeepersblog.wordpress.com/some-points-which-i-wish-all-my-new-prospective-students-know/

–
Some books which are must read. I tell all my friends and students to read these
You should read the books by Daniel Kahneman,
https://vk.com/doc23267904_175119602
The Black Swan – by Nassim Taleb
http://shifter-magazine.com/wp-content/uploads/2015/02/Taleb_The-Black-Swan.pdf
also
see http://stavochka.com/files/Nate_Silver_The_Signal_and_the_Noise.pdf
Nudge by Thaler and Sunstein
https://ethicslab.georgetown.edu/studio/wordpress/wp-content/uploads/2015/02/Richard_H._Thaler_Cass_R._Sunstein_Nudge_Impro_BookFi.org_.pdf
book which explains pricing is ” The undercover Economist “
or
http://ebook.stepor.com/book/the-undercover-economist-76396-pdf.html
–
Many more free pdf e-Books are available at ( such as H C Verma Concepts of Physics Solutions, Arihant Books, free download eBooks for IIT JEE guides, AIEEE IIT JEE advanced Chapter wise solutions, preparation materials )
1 ] A Guide Book to Mechanism in Organic Chemistry by Peter Sykes
A_GUIDE_BOOK_TO_MECHANISM_IN_ORGANIC_CHEMISTRY
2 ] Nomenclature of Inorganic Chemistry – IUPAC Recommendations 2005
Nomenclature of Inorganic Chemistry – IUPAC Recommendations 2005
3 ] Linear Algebra For Dummies
4 ] Calculus Workbook For Dummies
5 ] Differential Equations For Dummies
Differential_Equations_For_Dummies
6 ] Linear Algebra by Jim Hefferon
7 ] Mathematics – Puzzles from around the world
Mathematics—Puzzles-from-around-the-world
8 ] Graph Theory by Reinhard Diestel
9 ] Electronics for Dummies
10 ] Electronics Projects for Dummies
Electronics Projects For Dummies
11 ] Physics For Dummies
12 ] Physics Workbook For Dummies
13 ] Inorganic Chemistry James E. House
Inorganic Chemistry James E. House
14 ] Inorganic Chemistry by Cox
15 ] Inorganic Chemistry 5th Edition Miessler
Inorganic Chemistry 5th Edition Miessler
16 ] Fundamentals of Organic Chemistry Solomon
Fundamentals of Organic Chemistry Solomon
17 ] Illustrated Guide to Home Chemistry Experiments
Illustrated Guide to Home Chemistry Experiments
:-{D
e-Book-e-Book-e-Book-e-Book-e-Book-e-Book-e-Book-e-Book-e-Book-e-Book–e-Book
If you want to sell your House, why do you have to pay 2% to a Broker or to a website ?
You can advertise for free to sell your House at free4u.info
Professor Subhashish Chattopadhyay is providing a Social Service for all in Bangalore, to advertise for Free
If you want to sell your Car, why do you have to pay 2% to a Broker or to a website ?
You can advertise for free to sell your Car at free4u.info
Professor Subhashish Chattopadhyay is providing a Social Service for all in Bangalore, to advertise for Free
If you are looking for Organ Donation, where do you ask ? Where do you want to put up your requirements ? Do you give costly ads ?
You can advertise or Post Classifieds for free at free4u.info
Professor Subhashish Chattopadhyay is providing a Social Service for all in Bangalore, to advertise for Free. Post all kinds of Classified ads and Requirements for FREE.
If you are a Tutor, or a Shopkeeper, or a Teacher, or a Cook, or a Gardener, or a Dog Trainer ….. or something something something….. How can you afford costly ads ? Post your requirements for free at free4u.info Advertise yourself free at free4u.info Doing a garage sell …. Tell all for free at free4u.info
Looking for a Nanny ? You can get Nannys in free4u.info
Nannys looking for jobs ? Want children to take care ? You get the child and Parents in free4u.info
Professor Subhashish Chattopadhyay is providing a Social Service for all in Bangalore, to advertise for Free. Post all kinds of Classified ads and Requirements for FREE. Following Categories and Subcategories will surely help you. This is not an exhaustive list. You can give general requirements as well. Post all your skills. Post all your needs. Looking for a job ? You can post your profile as well.
Some call this as ” Yellow Pages “. free4u.info
Some call this a FREE listing sites free4u.info
–








Bike ( Want to sell your Bike ? Want a Buyer ? Advertise for free at free4u.info )
–
–
Solutions to Chapter 3 :

Classification of Elements and Periodicity in Properties Problem

🙂
Must see https://zookeepersblog.wordpress.com/some-points-which-i-wish-all-my-new-prospective-students-know/
🙂
xxxxxxxxxxxxxxxxxxxxxxxxxx
The next chapter Solution is at https://zookeepersblog.wordpress.com/ncert-cbse-standard-11-chemistry-chapter-4-chemical-bonding-and-molecular-structure/
!
The previous chapter Solution is at https://zookeepersblog.wordpress.com/ncert-cbse-standard-11-chemistry-chapter-2-structure-of-atom/
!
xxxxxxxxxxxxxxxxxxxxxxxxxx

🙂
Periodic Properties across a period and Group

🙂

🙂
Gyan Question

Ans ( b )

🙂


🙂
–
–
–
–
–
Gyan
Pauling Scale of Electronegativity

🙂
Gyan Question

Ans ( c )

🙂


🙂
Gyan Question

Ans ( b )

🙂

Gyan Question

Ans : ( c )

🙂
Gyan Question :
What are typical Elements ?

🙂
Gyan Question

Ans ( b )
The removal of an electron from Mg will require higher energy due to higher Nuclear charge
🙂


🙂
Gyan Question

Ans ( c )

🙂
Gyan Question

🙂
Gyan Question

Ans ( b )

🙂

🙂
Gyan Question

Ans ( c )
Boron has highest melting point due to icosahedral ( 20 faced ) structure with Boron atoms at all 12 corners.


🙂

🙂
Gyan Question

🙂
Gyan Question

Ans ( b )

🙂
Gyan Question :

🙂
Gyan Question :

🙂

🙂
Gyan Question :


🙂

🙂
Gyan Question :


🙂

🙂
Gyan Question

🙂

🙂

🙂
Gyan Question :

🙂

🙂
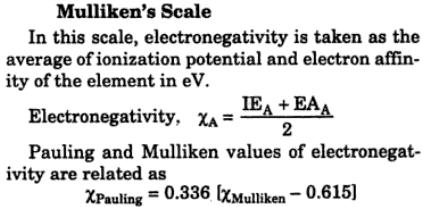
🙂

🙂
Gyan Question :

🙂

🙂
Gyan Question :

🙂

🙂
Gyan Question :


🙂

🙂
Gyan Question :


🙂

🙂
Gyan Question :



🙂

🙂
Gyan Question :


🙂

🙂
Gyan Question :


🙂
Gyan Question :
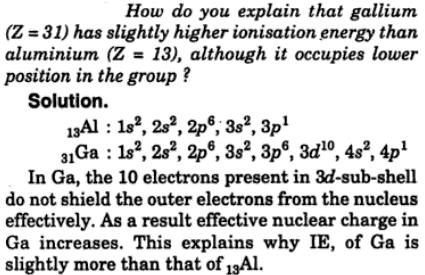
🙂
Gyan Question :
What are Bridge Elements ?

🙂
Gyan Question :


🙂
Gyan Question

🙂
Gyan

🙂

🙂
Gyan Question :

🙂
Gyan

🙂

🙂
Gyan Question on plot of Ionization Energy

Ans : ( c )

🙂
Gyan Question :

🙂
Gyan Question

Ans ( d )
🙂
Gyan Question :

🙂
Gyan Question

Ans ( c )

🙂

🙂
Gyan Question

Ans ( c )

🙂
Gyan Question :
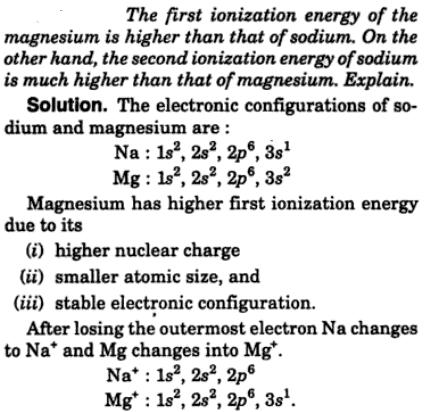

🙂
Electronic Configuration of Nitrogen and Oxygen

Gyan Question

Ans ( c )

🙂
Gyan Question :


🙂
Gyan Question

Ans ( b )

🙂

🙂
Gyan Question :

🙂
Gyan Question

Ans ( a )
The Electronic configuration of Na, Mg, Al, and Si

🙂
Gyan Question

🙂
Gyan Question

Ans ( a )

🙂

🙂
Gyan Question
The ionic radii of Cl-, S2-, P3-

Ans ( b )
🙂
Electron Affinity Gyan


🙂
Gyan Question

Ans ( a )
The Enthalpy of sublimation decreases descending down the group.
🙂
Gyan Question
The ionic radii of Na+, Mg2+ and Al3+

Ans ( a )
🙂
Gyan Question :

🙂
Gyan Question on density
Which of the following elements are expected to have least density

Ans ( d )

🙂

🙂
Gyan Question

Ans ( a )

Gyan Question

Ans ( a )
With increase in atomic number , size generally decreases but due to larger electronic repulsion the reverse of this is observed in 45Rh < 46Pd < 47Ag
🙂
Gyan Question :


🙂
Gyan Question

Ans ( d )
Inert gases have octet full. The change is maximum from Group 17 to Group 18
🙂

🙂
Gyan Question

Ans ( d )

Gyan Question

Ans : ( b )
Li+ , Be 2+, and B3+ are isoelectronic. Their size decreases with increasing atomic number.
🙂

Gyan Question

Ans ( b )

Gyan Question

Ans ( a )
The change is maximum while going from Group 1 to Group 2. As electron is added to same orbital.
xxxxxxxxxxxxxxxxxxxxxxxxxx
The next chapter Solution is at https://zookeepersblog.wordpress.com/ncert-cbse-standard-11-chemistry-chapter-4-chemical-bonding-and-molecular-structure/
!
The previous chapter Solution is at https://zookeepersblog.wordpress.com/ncert-cbse-standard-11-chemistry-chapter-2-structure-of-atom/
!
xxxxxxxxxxxxxxxxxxxxxxxxxx
Gyan Question

Ans ( a )
For an isoelectronic species the size increases with more negative charge
Gyan Question

Ans ( a )
Gyan Question

Ans ( c )
Gyan Question

Ans ( c )
xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx
The Periodic Table is arguably the most important concept in chemistry, both in principle and in practice. It is the everyday support for students, it suggests new avenues of research to professionals, and it provides a succinct organization of the whole of chemistry. It is a remarkable demonstration of the fact that the chemical elements are not a random cluster of entities but instead display trends and lie together in families. An awareness of the Periodic Table is essential to anyone who wishes to disentangle the world and see how it is built up from the fundamental building blocks of the chemistry, the chemical elements.
Glenn T. Seaborg
In this Unit, we will study the historical development of the Periodic Table as it stands today and the Modern Periodic Law. We will also learn how the periodic classification follows as a logical consequence of the electronic configuration of atoms. Finally, we shall examine some of the periodic trends in the physical and chemical properties of the elements.
3.1 WHY DO WE NEED TO CLASSIFY ELEMENTS ?
We know by now that the elements are the basic units of all types of matter. In 1800, only 31 elements were known. By 1865, the number of identified elements had more than doubled to 63. At present 114 elements are known. Of them, the recently discovered elements are man-made. Efforts to synthesise new elements are continuing. With such a large number of elements it is very difficult to study individually the chemistry of all these elements and their innumerable compounds individually. To ease out this problem, scientists searched for a systematic way to organise their knowledge by classifying the elements. Not only that it would rationalize known chemical facts about elements, but even predict new ones for undertaking further study.
3.2 GENESIS OF PERIODIC CLASSIFICATION
Classification of elements into groups and development of Periodic Law and Periodic Table are the consequences of systematising the knowledge gained by a number of scientists through their observations and experiments. The German chemist, Johann Dobereiner in early 1800’s was the first to consider the idea of trends among properties of elements. By 1829 he noted a similarity among the physical and chemical properties of several groups of three elements (Triads). In each case, he noticed that the middle element of each of the Triads had an atomic weight about half way between the atomic weights of the other two (Table 3.1). Also the properties of the middle element were in between those of the other two members. Since Dobereiner’s relationship, referred to as the Law of Triads, seemed to work only for a few elements, it was dismissed as coincidence. The next reported attempt to classify elements was made by a French geologist, A.E.B. de Chancourtois in 1862. He arranged the then known elements in order of increasing atomic weights and made a cylindrical table of elements to display the periodic recurrence of properties. This also did not attract much attention. The English chemist, John Alexander Newlands in 1865 profounded the Law of Octaves. He arranged the elements in increasing order of their atomic weights and noted that every eighth element had properties similar to the first element (Table 3.2). The relationship was just like every eighth note that resembles the first in octaves of music. Newlands’s Law of Octaves seemed to be true only for elements up to calcium.Although his idea was not widely accepted at that time, he, for his work, was later awarded Davy Medal in 1887 by the Royal Society, London.
The Periodic Law, as we know it today owes its development to the Russian chemist, Dmitri Mendeleev (1834-1907) and the German chemist, Lothar Meyer (1830-1895). Working independently, both the chemists in 1869 proposed that on arranging elements in the increasing order of their atomic weights, similarities appear in physical and chemical properties at regular intervals. Lothar Meyer plotted the physical properties such as atomic volume, melting point and boiling point against atomic weight and obtained a periodically repeated pattern. Unlike Newlands, Lothar Meyer observed a change in length of that repeating pattern. By 1868, Lothar Meyer had developed a table of the elements that closely resembles the Modern Periodic Table. However, his work was not published until after the work of Dmitri Mendeleev, the scientist who is generally credited with the development of the Modern Periodic Table.🙂
While Dobereiner initiated the study of periodic relationship, it was Mendeleev who was responsible for publishing the Periodic Law for the first time. It states as follows :
🙂
The properties of the elements are a periodic function of their atomic weights.
Mendeleev arranged elements in horizontal rows and vertical columns of a table in order of their increasing atomic weights in such a way that the elements with similar properties occupied the same vertical column or group. Mendeleev’s system of classifying elements was more elaborate than that of Lothar Meyer’s. He fully recognized the significance of periodicity and used broader range of physical and chemical properties to classify the elements. In particular, Mendeleev relied on the similarities in the empirical formulas and properties of the compounds formed by the elements. He realized that some of the elements did not fit in with his scheme of classification if the order of atomic weight was strictly followed. He ignored the order of atomic weights, thinking that the atomic measurements might be incorrect, and placed the elements with similar properties together. For example, iodine with lower atomic weight than that of tellurium (Group VI) was placed in Group VII along with fluorine, chlorine, bromine because of similarities in properties (Fig. 3.1). At the same time, keeping his primary aim of arranging the elements of similar properties in the same group, he proposed that some of the elements were still undiscovered and, therefore, left several gaps in the table. For example, both gallium and germanium were unknown at the time Mendeleev published his Periodic Table. He left the gap under aluminium and a gap under silicon, and called these elements Eka- Aluminium and Eka-Silicon. Mendeleev predicted not only the existence of gallium and germanium, but also described some of their general physical properties. These elements were discovered later. Some of the properties predicted by Mendeleev for these elements and those found experimentally are listed in Table 3.3.
The boldness of Mendeleev’s quantitative predictions and their eventual success made him and his Periodic Table famous. Mendeleev’s Periodic Table published in 1905 is shown in Fig. 3.1.
_____________________________________________________________________________________________________________________
Dmitri Ivanovich Mendeleev ( 1834 – 1907 )
🙂
Dmitri Mendeleev was born in Tobalsk, Siberia in Russia. After his father’s death, the family moved to St. Petersburg. He received his Master’s degree in Chemistry in 1856 and the doctoral degree in 1865. He taught at the University of St.Petersburg where he was appointed Professor of General Chemistry in 1867. Preliminary work for his great textbook “Principles of Chemistry” led Mendeleev to propose the Periodic Law and to construct his Periodic Table of elements. At that time, the structure of atom was unknown and Mendeleev’s idea to consider that the properties of the elements were in someway related to their atomic masses was a very imaginative one. To place certain elements into the correct group from the point of view of their chemical properties, Mendeleev reversed the order of some pairs of elements and asserted that their atomic masses were incorrect. Mendeleev also had the foresight to leave gaps in the Periodic Table for elements unknown at that time and predict their properties from the trends that he observed among the properties of related elements. Mendeleev’s predictions were proved to be astonishingly correct when these elements were discovered later.
Mendeleev’s Periodic Law spurred several areas of research during the subsequent decades. The discovery of the first two noble gases helium and argon in 1890 suggested the possibility that there must be other similar elements to fill an entire family. This idea led Ramsay to his successful search for krypton and xenon. Work on the radioactive decay series for uranium and thorium in the early years of twentieth century was also guided by the Periodic Table.
Mendeleev was a versatile genius. He worked on many problems connected with Russia’s natural resources. He invented an accurate barometer. In 1890, he resigned from the Professorship. He was appointed as the Director of the Bureau of Weights and Measures. He continued to carry out important research work in many areas until his death in 1907.
You will notice from the modern Period Table (Fig. 3.2) that Mendeleev’s name has been immortalized by naming the element with atomic number 101, as Mendelevium. This name was proposed by American scientist Glenn T. Seaborg, the discoverer of this element, “in recognition of the pioneering role of the great Russian Chemist who was the first to use the periodic system of elements to predict the chemical properties of undiscovered elements, a principle which has been the key to the discovery of nearly all the transuranium elements”. ____________________________________________________________________________________________________________________
3.3 MODERN PERIODIC LAW AND THE PRESENT FORM OF THE PERIODIC TABLE
We must bear in mind that when Mendeleev developed his Periodic Table, chemists knew nothing about the internal structure of atom. However, the beginning of the 20th century witnessed profound developments in theories about sub-atomic particles. In 1913, the English physicist, Henry Moseley observed regularities in the characteristic X-ray spectra of the elements. A plot of √ν (where ν is frequency of X-rays emitted) against atomic number (Z ) gave a straight line and not the plot of √ν vs atomic mass. He thereby showed that the atomic number is a more fundamental property of an element than its atomic mass. Mendeleev’s Periodic Law was, therefore, accordingly modified. This is known as the Modern Periodic Law and can be stated as :
The physical and chemical properties of the elements are periodic functions of their atomic numbers.
The Periodic Law revealed important analogies among the 94 naturally occurring elements (neptunium and plutonium like actinium and protoactinium are also found in pitch blende – an ore of uranium). It stimulated renewed interest in Inorganic Chemistry and has carried into the present with the creation of artificially produced short-lived elements.
You may recall that the atomic number is equal to the nuclear charge (i.e., number of protons) or the number of electrons in a neutral atom. It is then easy to visualize the significance of quantum numbers and electronic configurations in periodicity of elements. In fact, it is now recognized that the Periodic Law is essentially the consequence of the periodic variation in electronic configurations, which indeed determine the physical and chemical properties of elements and their compounds.
Numerous forms of Periodic Table have been devised from time to time. Some forms emphasise chemical reactions and valence, whereas others stress the electronic configuration of elements. A modern version, the so-called “long form” of the Periodic Table of the elements (Fig. 3.2), is the most convenient and widely used. The horizontal rows (which Mendeleev called series) are called periods and the vertical columns, groups. Elements having similar outer electronic configurations in their atoms are arranged in vertical columns, referred to as groups or families. According to the recommendation of International Union of Pure and Applied Chemistry (IUPAC), the groups are numbered from 1 to 18 replacing the older notation of groups IA… VIIA, VIII, IB … VIIB and 0.
🙂
There are altogether seven periods. The period number corresponds to the highest principal quantum number (n) of the elements in the period. The first period contains 2 elements. The subsequent periods consists of 8, 8, 18, 18 and 32 elements, respectively. The seventh period is incomplete and like the sixth period would have a theoretical maximum (on the basis of quantum numbers) of 32 elements. In this form of the Periodic Table, 14 elements of both sixth and seventh periods (lanthanoids and actinoids, respectively) are placed in separate panels at the bottom*.
————————————————————————————————————————————–
* Glenn T. Seaborg’s work in the middle of the 20th century starting with the discovery of plutonium in 1940, followed by those of all the transuranium elements from 94 to 102 led to reconfiguration of the periodic table placing the actinoids below the lanthanoids. In 1951, Seaborg was awarded the Nobel Prize in chemistry for his work. Element 106 has been named Seaborgium (Sg) in his honour. —————————————————————————————————————————————-
3.4 NOMENCLATURE OF ELEMENTS WITH ATOMIC NUMBERS > 100
The naming of the new elements had been traditionally the privilege of the discoverer (or discoverers) and the suggested name was ratified by the IUPAC. In recent years this has led to some controversy. The new elements with very high atomic numbers are so unstable that only minute quantities, sometimes only a few atoms of them are obtained. Their synthesis and characterisation, therefore, require highly sophisticated costly equipment and laboratory. Such work is carried out with competitive spirit only in some laboratories in the world. Scientists, before collecting the reliable data on the new element, at times get tempted to claim for its discovery. For example, both American and Soviet scientists claimed credit for discovering element 104. The Americans named it Rutherfordium whereas Soviets named it Kurchatovium. To avoid such problems, the IUPAC has made recommendation that until a new element’s discovery is proved, and its name is officially recognized, a systematic nomenclature be derived directly from the atomic number of the element using the numerical roots for 0 and numbers 1-9. These are shown in Table 3.4. The roots are put together in order of digits which make up the atomic number and “ium” is added at the end. The IUPAC names for elements with Z above 100 are shown in Table 3.5.
🙂
🙂
Thus, the new element first gets a temporary name, with symbol consisting of three letters. Later permanent name and symbol are given by a vote of IUPAC representatives from each country. The permanent name might reflect the country (or state of the country) in which the element was discovered, or pay tribute to a notable scientist. As of now, elements with atomic numbers up to 112, 114 and 116 have been discovered. Elements with atomic numbers 113, 115, 117 and 118 are not yet known.
Problem 3.1
What would be the IUPAC name and symbol for the element with atomic number 120?
Solution
From Table 3.4, the roots for 1, 2 and 0 are un, bi and nil, respectively. Hence, the symbol and the name respectively are Ubn and unbinilium.
3.5 ELECTRONIC CONFIGURATIONS OF ELEMENTS AND THE PERIODIC TABLE
In the preceding unit we have learnt that an electron in an atom is characterised by a set of four quantum numbers, and the principal quantum number (n ) defines the main energy level known as shell. We have also studied about the filling of electrons into different subshells, also referred to as orbitals (s, p, d, f ) in an atom. The distribution of electrons into orbitals of an atom is called its electronic configuration. An element’s location in the Periodic Table reflects the quantum numbers of the last orbital filled. In this section we will observe a direct connection between the electronic configurations of the elements and the long form of the Periodic Table.
(a) Electronic Configurations in Periods
The period indicates the value of n for the outermost or valence shell. In other words, successive period in the Periodic Table is associated with the filling of the next higher principal energy level (n = 1, n = 2, etc.). It can be readily seen that the number of elements in each period is twice the number of atomic orbitals available in the energy level that is being filled. The first period (n = 1) starts with the filling of the lowest level (1s) and therefore has two elements – hydrogen (ls1) and helium (ls2) when the first shell (K) is completed. The second period (n = 2) starts with lithium and the third electron enters the 2s orbital. The next element, beryllium has four electrons and has the electronic configuration 1s22s2. Starting from the next element boron, the 2p orbitals are filled with electrons when the L shell is completed at neon (2s22p6). Thus there are 8 elements in the second period. The third period (n = 3) begins at sodium, and the added electron enters a 3s orbital. Successive filling of 3s and 3p orbitals gives rise to the third period of 8 elements from sodium to argon. The fourth period (n = 4) starts at potassium, and the added electrons fill up the 4s orbital. Now you may note that before the 4p orbital is filled, filling up of 3d orbitals becomes energetically favourable and we come across the so called 3d transition series of elements. This starts from scandium (Z = 21) which has the electronic configuration 3d14s2. The 3d orbitals are filled at zinc (Z=30) with electronic configuration 3d104s2 . The fourth period ends at krypton with the filling up of the 4p orbitals. Altogether we have 18 elements in this fourth period. The fifth period (n = 5) beginning with rubidium is similar to the fourth period and contains the 4d transition series starting at yttrium (Z = 39). This period ends at xenon with the filling up of the 5p orbitals. The sixth period (n = 6) contains 32 elements and successive electrons enter 6s, 4f, 5d and 6p orbitals, in the order – filling up of the 4f orbitals begins with cerium (Z = 58) and ends at lutetium (Z = 71) to give the 4f-inner transition series which is called the lanthanoid series. The seventh period (n = 7) is similar to the sixth period with the successive filling up of the 7s, 5f, 6d and 7p orbitals and includes most of the man-made radioactive elements. This period will end at the element with atomic number 118 which would belong to the noble gas family. Filling up of the 5f orbitals after actinium (Z = 89) gives the 5f-inner transition series known as the actinoid series. The 4f-and 5f-inner transition series of elements are placed separately in the Periodic Table to maintain its structure and to preserve the principle of classification by keeping elements with similar properties in a single column.
Problem 3.2
How would you justify the presence of 18 elements in the 5th period of the Periodic Table?
Solution
When n = 5, l = 0, 1, 2, 3. The order in which the energy of the available orbitals 4d, 5s and 5p increases is 5s < 4d < 5p. The total number of orbitals available are 9. The maximum number of electrons that can be accommodated is 18; and therefore 18 elements are there in the 5th period.
(b) Groupwise Electronic Configurations
Elements in the same vertical column or group have similar valence shell electronic configurations, the same number of electrons in the outer orbitals, and similar properties. For example, the Group 1 elements (alkali metals) all have ns1 valence shell electronic configuration as shown below.
Thus it can be seen that the properties of an element have periodic dependence upon its atomic number and not on relative atomic mass.
Gyan Question
Ans ( c )
🙂
Ans ( b )
Sulphur has the highest value of electron affinity.
🙂
3.6 ELECTRONIC CONFIGURATIONS AND TYPES OF ELEMENTS: s-, p-, d-, f- BLOCKS
The aufbau (build up) principle and the electronic configuration of atoms provide a theoretical foundation for the periodic classification. The elements in a vertical column of the Periodic Table constitute a group or family and exhibit similar chemical behaviour. This similarity arises because these elements have the same number and same distribution of electrons in their outermost orbitals. We can classify the elements into four blocks viz., s-block, p-block, d-block and f-block depending on the type of atomic orbitals that are being filled with electrons. This is illustrated in Fig. 3.3. We notice two exceptions to this categorisation. Strictly, helium belongs to the s-block but its positioning in the p-block along with other group 18 elements is justified because it has a completely filled valence shell (1s2) and as a result, exhibits properties characteristic of other noble gases. The other exception is hydrogen. It has a lone s-electron and hence can be placed in group 1 (alkali metals). It can also gain an electron to achieve a noble gas arrangement and hence it can behave similar to a group 17 (halogen family) elements. Because it is a special case, we shall place hydrogen separately at the top of the Periodic Table as shown in Fig. 3.2 and Fig. 3.3. We will briefly discuss the salient features of the four types of elements marked in the Periodic Table. More about these elements will be discussed later. During the description of their features certain terminology has been used which has been classified in section 3.7.
🙂
🙂
3.6.1 The s-Block Elements
The elements of Group 1 (alkali metals) and Group 2 (alkaline earth metals) which have ns1 and ns2 outermost electronic configuration belong to the s-Block Elements. They are all reactive metals with low ionization enthalpies. They lose the outermost electron(s) readily to form 1+ ion (in the case of alkali metals) or 2+ ion (in the case of alkaline earth metals). The metallic character and the reactivity increase as we go down the group. Because of high reactivity they are never found pure in nature. The compounds of the s-block elements, with the exception of those of lithium and beryllium are predominantly ionic.
3.6.2 The p-Block Elements
The p-Block Elements comprise those belonging to Group 13 to 18 and these together with the s-Block Elements are called the epresentative Elements or Main Group Elements. The outermost electronic configuration varies from ns2np1 to ns2np6 in each period. At the end of each period is a noble gas element with a closed valence shell ns2np6 configuration. All the orbitals in the valence shell of the noble gases are completely filled by electrons and it is very difficult to alter this stable arrangement by the addition or removal of electrons. The noble gases thus exhibit very low chemical reactivity. Preceding the noble gas family are two chemically important groups of non-metals. They are the halogens (Group 17) and the chalcogens (Group 16). These two groups of elements have high negative electron gain enthalpies and readily add one or two electrons respectively to attain the stable noble gas configuration. The non-metallic character increases as we move from left to right across a period and metallic character increases as we go down the group.
3.6.3 The d-Block Elements (Transition Elements)
These are the elements of Group 3 to 12 in the centre of the Periodic Table. These are characterised by the filling of inner d orbitals by electrons and are therefore referred to as d-Block Elements. These elements have the general outer electronic configuration (n-1)d1-10ns0-2 . They are all metals. They mostly form coloured ions, exhibit variable valence (oxidation states), paramagnetism and oftenly used as catalysts. However, Zn, Cd and Hg which have the electronic configuration, (n-1)d10ns2 do not show most of the properties of transition elements. In a way, transition metals form a bridge between the chemically active metals of s-block elements and the less active elements of Groups 13 and 14 and thus take their familiar name “Transition Elements”.
3.6.4 The f-Block Elements (Inner-Transition Elements)
The two rows of elements at the bottom of the Periodic Table, called the Lanthanoids, Ce(Z = 58) – Lu(Z = 71) and Actinoids, Th(Z = 90) – Lr (Z = 103) are characterised by the outer electronic configuration (n-2)f1-14 (n-1)d0-1ns2. The last electron added to each element is filled in f- orbital. These two series of elements are hence called the Inner- Transition Elements (f-Block Elements). They are all metals. Within each series, the properties of the elements are quite similar. The chemistry of the early actinoids is more complicated than the corresponding lanthanoids, due to the large number of oxidation states possible for these actinoid elements. Actinoid elements are radioactive. Many of the actinoid elements have been made only in nanogram quantities or even less by nuclear reactions and their chemistry is not fully studied. The elements after uranium are called Transuranium Elements.
Problem 3.3
The elements Z = 117 and 120 have not yet been discovered. In which family / group would you place these elements and also give the electronic configuration in each case.
Solution
We see from Fig. 3.2, that element with Z = 117, would belong to the halogen family (Group 17) and the electronic configuration would be [Rn] 5f146d107s27p5. The element with Z = 120, will be placed in Group 2 (alkaline earth metals), and will have the electronic configuration [Uuo]8s2.
3.6.5 Metals, Non-metals and Metalloids
In addition to displaying the classification of elements into s-, p-, d-, and f-blocks, Fig. 3.3 shows another broad classification of elements based on their properties. The elements can be divided into Metals and Non-Metals. Metals comprise more than 78% of all known elements and appear on the left side of the Periodic Table. Metals are usually solids at room temperature [mercury is an exception; gallium and caesium also have very low melting points (303K and 302K, respectively)]. Metals usually have high melting and boiling points. They are good conductors of heat and electricity. They are malleable (can be flattened into thin sheets by hammering) and ductile (can be drawn into wires). In contrast, non-metals are located at the top right hand side of the Periodic Table. In fact, in a horizontal row, the property of elements change from metallic on the left to non-metallic on the right. Non-metals are usually solids or gases at room temperature with low melting and boiling points (boron and carbon are exceptions). They are poor conductors of heat and electricity. Most nonmetallic solids are brittle and are neither malleable nor ductile. The elements become more metallic as we go down a group; the nonmetallic character increases as one goes from left to right across the Periodic Table. The change from metallic to non-metallic character is not abrupt as shown by the thick zig-zag line in Fig. 3.3. The elements (e.g., silicon, germanium, arsenic, antimony and tellurium) bordering this line and running diagonally across the Periodic Table show properties that are characteristic of both metals and nonmetals. These elements are called Semi-metals or Metalloids.
Problem 3.4
Considering the atomic number and position in the periodic table, arrange the following elements in the increasing order of metallic character : Si, Be, Mg, Na, P.
Solution
Metallic character increases down a group and decreases along a period as we move from left to right. Hence the order of increasing metallic character is: P < Si < Be < Mg < Na.
3.7 PERIODIC TRENDS IN PROPERTIES OF ELEMENTS
There are many observable patterns in the physical and chemical properties of elements as we descend in a group or move across a period in the Periodic Table. For example, within a period, chemical reactivity tends to be high in Group 1 metals, lower in elements towards the middle of the table, and increases to a maximum in the Group 17 non-metals. Likewise within a group of representative metals (say alkali metals) reactivity increases on moving down the group, whereas within a group of non-metals (say halogens), reactivity decreases down the group. But why do the properties of elements follow these trends? And how can we explain periodicity? To answer these questions, we must look into the theories of atomic structure and properties of the atom. In this section we shall discuss the periodic trends in certain physical and chemical properties and try to explain them in terms of number of electrons and energy levels.
3.7.1 Trends in Physical Properties
There are numerous physical properties of elements such as melting and boiling points, heats of fusion and vaporization, energy of atomization, etc. which show periodic variations. However, we shall discuss the periodic trends with respect to atomic and ionic radii, ionization enthalpy, electron gain enthalpy and lectronegativity.
( a ) Atomic Radius
You can very well imagine that finding the size of an atom is a lot more complicated than measuring the radius of a ball. Do you know why? Firstly, because the size of an atom (~ 1.2 Å i.e., 1.2 x 10-10 m in radius) is very small. Secondly, since the electron cloud surrounding the atom does not have a sharp boundary, the determination of the atomic size cannot be precise. In other words, there is no practical way by which the size of an individual atom can be measured. However, an estimate of the atomic size can be made by knowing the distance between the atoms in the combined state. One practical approach to estimate the size of an atom of a non-metallic element is to measure the distance between two atoms when they are bound together by a single bond in a covalent molecule and from this value, the “Covalent Radius” of the element can be calculated. For example, the bond distance in the chlorine molecule (Cl2) is 198 pm and half this distance (99 pm), is taken as the atomic radius of chlorine. For metals, we define the term “Metallic Radius” which is taken as half the internuclear distance separating the metal cores in the metallic crystal. For example, the distance between two adjacent copper atoms in solid copper is 256 pm; hence the metallic radius of copper is assigned a value of 128 pm. For simplicity, in this book, we use the term Atomic Radius to refer to both covalent or metallic radius depending on whether the element is a non-metal or a metal. Atomic radii can be measured by X-ray or other spectroscopic methods.
The atomic radii of a few elements are listed in Table 3.6 . Two trends are obvious. We can explain these trends in terms of nuclear charge and energy level. The atomic size generally decreases across a period as illustrated in Fig. 3.4(a) for the elements of the second period. It is because within the period the outer electrons are in the same valence shell and the effective nuclear charge increases as the atomic number increases resulting in the increased attraction of electrons to the nucleus. Within a family or vertical column of the periodic table, the atomic radius increases regularly with atomic number as illustrated in Fig. 3.4(b). For alkali metals and halogens, as we descend the groups, the principal quantum number (n) increases and the valence electrons are farther from the nucleus. This happens because the inner energy levels are filled with electrons, which serve to shield the outer electrons from the pull of the nucleus. Consequently the size of the atom increases as reflected in the atomic radii.
🙂
🙂
Note that the atomic radii of noble gases are not considered here. Being monoatomic, their (non-bonded radii) values are very large. In fact radii of noble gases should be compared not with the covalent radii but with the van der Waals radii of other elements.
🙂
🙂
(b) Ionic Radius The removal of an electron from an atom results in the formation of a cation, whereas gain of an electron leads to an anion. The ionic radii can be estimated by measuring the distances between cations and anions in ionic crystals. In general, the ionic radii of elements exhibit the same trend as the atomic radii. A cation is smaller than its parent atom because it has fewer electrons while its nuclear charge remains the same. The size of an anion will be larger than that of the parent atom because the addition of one or more electrons would result in increased repulsion among the electrons and a decrease in effective nuclear charge. For example, the ionic radius of fluoride ion (F–) is 136 pm whereas the atomic radius of fluorine is only 64 pm. On the other hand, the atomic radius of sodium is 186 pm compared to the ionic radius of 95 pm for Na+.
When we find some atoms and ions which contain the same number of electrons, we call them isoelectronic species. For example, O2-, F–, Na+ and Mg2+ have the same number of electrons (10). Their radii would be different because of their different nuclear charges. The cation with the greater positive charge will have a smaller radius because of the greater attraction of the electrons to the nucleus. Anion with the greater negative charge will have the larger radius. In this case, the net repulsion of the electrons will outweigh the nuclear charge and the ion will expand in size.
Problem 3.5
Which of the following species will have the largest and the smallest size? Mg, Mg2+, Al, Al3+ .
Solution
Atomic radii decrease across a period. Cations are smaller than their parent atoms. Among isoelectronic species, the one with the larger positive nuclear charge will have a smaller radius. Hence the largest species is Mg; the smallest one is Al3+.
🙂
Ans : ( b, c, d )
🙂
(c) Ionization Enthalpy
A quantitative measure of the tendency of an element to lose electron is given by its Ionization Enthalpy. It represents the energy required to remove an electron from an isolated gaseous atom (X) in its ground state. In other words, the first ionization enthalpy for an element X is the enthalpy change (Δi H) for the reaction depicted in equation 3.1.
X(g) → X+(g) + e– (3.1)
The ionization enthalpy is expressed in units of kJ mol-1. We can define the second ionization enthalpy as the energy required to remove the second most loosely bound electron; it is the energy required to carry out the reaction shown in equation 3.2.
X+(g) → X2+(g) + e– (3.2)
Energy is always required to remove electrons from an atom and hence ionization enthalpies are always positive. The second ionization enthalpy will be higher than the first ionization enthalpy because it is more difficult to remove an electron from a positively charged ion than from a neutral atom. In the same way the third ionization enthalpy will be higher than the second and so on. The term “ionization enthalpy”, if not qualified, is taken as the first ionization enthalpy.
🙂
Ans : ( b, c )
🙂
The first ionization enthalpies of elements having atomic numbers up to 60 are plotted in Fig. 3.5. The periodicity of the graph is quite striking. You will find maxima at the noble gases which have closed electron shells and very stable electron configurations. On the other hand, minima occur at the alkali metals and their low ionization enthalpies can be correlated with their high reactivity. In addition, you will notice two trends the first ionization enthalpy generally increases as we go across a period and decreases as we descend in a group. These trends are illustrated in Figs. 3.6(a) and 3.6(b) respectively for the elements of the second period and the first group of the periodic table. You will appreciate that the ionization enthalpy and atomic radius are closely related properties. To understand these trends, we have to consider two factors : (i) the attraction of electrons towards the nucleus, and (ii) the repulsion of electrons from each other. The effective nuclear charge experienced by a valence electron in an atom will be less than the actual charge on the nucleus because of “shielding” or “screening” of the valence electron from the nucleus by the intervening core electrons. For example, the 2s electron in lithium is shielded from the nucleus by the inner core of 1s electrons. As a result, the valence electron experiences a net positive charge which is less than the actual charge of +3. In general, shielding is effective when the orbitals in the inner shells are completely filled. This situation occurs in the case of alkali metals which have a lone ns – outermost electron preceded by a noble gas electronic configuration.
🙂
🙂
🙂
Ans : ( b, c, d )
🙂
When we move from lithium to fluorine across the second period, successive electrons are added to orbitals in the same principal quantum level and the shielding of the nuclear charge by the inner core of electrons does not increase very much to compensate for the increased attraction of the electron to the nucleus. Thus, across a period, increasing nuclear charge outweighs the shielding. Consequently, the outermost electrons are held more and more tightly and the ionization enthalpy increases across a period. As we go down a group, the outermost electron being increasingly farther from the nucleus, there is an increased shielding of the nuclear charge by the electrons in the inner levels. In this case, increase in shielding outweighs the increasing nuclear charge and the removal of the outermost electron requires less energy down a group.
🙂
Ans : ( a, b )
🙂
From Fig. 3.6(a), you will also notice that the first ionization enthalpy of boron (Z = 5) is slightly less than that of beryllium (Z = 4) even though the former has a greater nuclear charge. When we consider the same principal quantum level, an s-electron is attracted to the nucleus more than a p-electron. In beryllium, the electron removed during the ionization is an s-electron whereas the electron removed during ionization of boron is a p-electron. The penetration of a 2s-electron to the nucleus is more than that of a 2p-electron; hence the 2p electron of boron is more shielded from the nucleus by the inner core of electrons than the 2s electrons of beryllium. Therefore, it is easier to remove the 2p-electron from boron compared to the removal of a 2s- electron from beryllium. Thus, boron has a smaller first ionization enthalpy than beryllium. Another “anomaly” is the smaller first ionization enthalpy of oxygen compared to nitrogen. This arises because in the nitrogen atom, three 2p-electrons reside in different atomic orbitals (Hund’s rule) whereas in the oxygen atom, two of the four 2p-electrons must occupy the same 2p-orbital resulting in an increased electron-electron repulsion. Consequently, it is easier to remove the fourth 2p-electron from oxygen than it is, to remove one of the three 2p-electrons from nitrogen.
🙂
Ans : ( a, c, d )
🙂
Problem 3.6
The first ionization enthalpy (Δi H ) values of the third period elements, Na, Mg and Si are respectively 496, 737 and 786 kJ mol-1. Predict whether the first Δi H value for Al will be more close to 575 or 760 kJ mol-1 ? Justify your answer.
Solution
It will be more close to 575 kJ mol-1. The value for Al should be lower than that of Mg because of effective shielding of 3p electrons from the nucleus by 3s-electrons.
(d) Electron Gain Enthalpy
When an electron is added to a neutral gaseous atom (X) to convert it into a negative ion, the enthalpy change accompanying the process is defined as the Electron Gain Enthalpy (ΔegH). Electron gain enthalpy provides a measure of the ease with which an atom adds an electron to form anion as represented by equation 3.3.
X(g) + e– → X–(g) (3.3)
Depending on the element, the process of adding an electron to the atom can be either endothermic or exothermic. For many elements energy is released when an electron is added to the atom and the electron gain enthalpy is negative. For example, group 17 elements (the halogens) have very high negative electron gain enthalpies because they can attain stable noble gas electronic configurations by picking up an electron. On the other hand, noble gases have large positive electron gain enthalpies because the electron has to enter the next higher principal quantum level leading to a very unstable electronic configuration. It may be noted that electron gain enthalpies have large negative values toward the upper right of the periodic table preceding the noble gases. The variation in electron gain enthalpies of elements is less systematic than for ionization enthalpies. As a general rule, electron gain enthalpy becomes more negative with increase in the atomic number across a period. The effective nuclear charge increases from left to right across a period and consequently it will be easier to add an electron to a smaller atom since the added electron on an average would be closer to the positively charged nucleus. We should also expect electron gain enthalpy to become less negative as we go down a group because the size of the atom increases and the added electron would be farther from the nucleus. This is generally the case (Table 3.7). However, electron gain enthalpy of O or F is less negative than that of the succeeding element. This is because when an electron is added to O or F, the added electron goes to the smaller n = 2 quantum level and suffers significant repulsion from the other electrons present in this level. For the n = 3 quantum level (S or Cl), the added electron occupies a larger region of space and the electron-electron repulsion is much less.
🙂
Ans : ( a,b, c, and d )
🙂
🙂
* In many books, the negative of the enthalpy change for the process depicted in equation 3.3 is defined as the ELECTRON AFFINITY (A<sub>e</sub> ) of the atom under consideration. If energy is released when an electron is added to an atom, the electron affinity is taken as positive, contrary to thermodynamic convention. If energy has to be supplied to add an electron to an atom, then the electron affinity of the atom is assigned a negative sign. However, electron affinity is defined as absolute zero and, therefore at any other temperature (T) heat capacities of the reactants and the products have to be taken into account in Δ<sub>eg</sub>H = -A<sub>e</sub> – 5/2 RT. ______________________________________________________________________________________________________________________
🙂
Ans : ( b,c )
🙂
Problem 3.7
Which of the following will have the most negative electron gain enthalpy and which the least negative? P, S, Cl, F. Explain your answer.
Solution
Electron gain enthalpy generally becomes more negative across a period as we move from left to right. Within a group, electron gain enthalpy becomes less negative down a group. However, adding an electron to the 2p-orbital leads to greater repulsion than adding an electron to the larger 3p-orbital. Hence the element with most negative electron gain enthalpy is chlorine; the one with the least negative electron gain enthalpy is phosphorus.
(e) Electronegativity
A qualitative measure of the ability of an atom in a chemical compound to attract shared electrons to itself is called electronegativity. Unlike ionization enthalpy and electron gain enthalpy, it is not a measureable quantity. However, a number of numerical scales of electronegativity of elements viz., Pauling scale, Mulliken-Jaffe scale, Allred-Rochow scale have been developed. The one which is the most widely used is the Pauling scale. Linus Pauling, an American scientist, in 1922 assigned arbitrarily a value of 4.0 to fluorine, the element considered to have the greatest ability to attract electrons. Approximate values for the electronegativity of a few elements are given in Table 3.8(a)
🙂
🙂
The electronegativity of any given element is not constant; it varies depending on the element to which it is bound. Though it is not a measurable quantity, it does provide a means of predicting the nature of force that holds a pair of atoms together – a relationship that you will explore later. Electronegativity generally increases across a period from left to right (say from lithium to fluorine) and decrease down a group (say from fluorine to astatine) in the periodic table. How can these trends be explained? Can the electronegativity be related to atomic radii, which tend to decrease across each period from left to right, but increase down each group ? The attraction between the outer (or valence) electrons and the nucleus increases as the atomic radius decreases in a period. The electronegativity also increases. On the same account electronegativity values decrease with the increase in atomic radii down a group. The trend is similar to that of ionization enthalpy. Knowing the relationship between electronegativity and atomic radius, can you now visualise the relationship between electronegativity and non-metallic properties? Non-metallic elements have strong tendency to gain electrons. Therefore, electronegativity is directly related to that non-metallic properties of elements. It can be further extended to say that the electronegativity is inversely related to the metallic properties of elements. Thus, the increase in electronegativities across a period is accompanied by an increase in non-metallic properties (or decrease in metallic properties) of elements. Similarly, the decrease in electronegativity down a group is accompanied by a decrease in non-metallic properties (or increase in metallic properties) of elements. All these periodic trends are summarized in figure 3.7.
🙂
🙂
3.7.2 Periodic Trends in Chemical Properties
Ans : ( a,b )
🙂
Most of the trends in chemical properties of elements, such as diagonal relationships, inert pair effect, effects of lanthanoid contraction etc. will be dealt with along the discussion of each group in later units. In this section we shall study the periodicity of the valence state shown by elements and the anomalous properties of the second period elements (from lithium to fluorine).
(a) Periodicity of Valence or Oxidation States
The valence is the most characteristic property of the elements and can be understood in terms of their electronic configurations. The valence of representative elements is usually (though not necessarily) equal to the number of electrons in the outermost orbitals and / or equal to eight minus the number of outermost electrons as shown below. Nowadays the term oxidation state is frequently used for valence. Consider the two oxygen containing compounds: OF2 and Na2O. The order of electronegativity of the three elements involved in these compounds is F > O > Na. Each of the atoms of fluorine, with outer electronic configuration 2s22p5, shares one electron with oxygen in the OF2 molecule. Being highest electronegative element, fluorine is given oxidation state -1. Since there are two fluorine atoms in this molecule, oxygen with outer electronic configuration 2s22p4 shares two electrons with fluorine atoms and thereby exhibits oxidation state +2. In Na2O, oxygen being more electronegative accepts two electrons, one from each of the two sodium atoms and, thus, shows oxidation state -2. On the other hand sodium with electronic configuration 3s1 loses one electron to oxygen and is given oxidation state +1. Thus, the oxidation state of an element in a particular compound can be defined as the charge acquired by its atom on the basis of electronegative consideration from other atoms in the molecule.
🙂
🙂
Ans : ( b, d )
🙂
Problem 3.8
Using the Periodic Table, predict the formulas of compounds which might be formed by the following pairs of elements; (a) silicon and bromine (b) aluminium and sulphur.
Solution
(a) Silicon is group 14 element with a valence of 4; bromine belongs to the halogen family with a valence of 1. Hence the formula of the compound formed would be SiBr4.
(b) Aluminium belongs to group 13 with a valence of 3; sulphur belongs to group 16 elements with a valence of 2. Hence, the formula of the compound formed would be Al2S3. Some periodic trends observed in the valence of elements (hydrides and oxides) are shown in Table 3.9. Other such periodic trends which occur in the chemical behaviour of the elements are discussed elsewhere in this book.
🙂
🙂
There are many elements which exhibit variable valence. This is particularly characteristic of transition elements and actinoids, which we shall study later.
🙂
Ans : ( a, c )
🙂
(b) Anomalous Properties of Second Period Elements
The first element of each of the groups 1 (lithium) and 2 (beryllium) and groups 13-17 (boron to fluorine) differs in many respects from the other members of their respective group. For example, lithium unlike other alkali metals, and beryllium unlike other alkaline earth metals, form compounds with pronounced covalent character; the other members of these groups predominantly form ionic compounds. In fact the behaviour of lithium and beryllium is more similar with the second element of the following group i.e., magnesium and aluminium, respectively. This sort of similarity is commonly referred to as diagonal relationship in the periodic properties.
🙂
🙂
What are the reasons for the different chemical behaviour of the first member of a group of elements in the s- and p-blocks compared to that of the subsequent members in the same group? The anomalous behaviour is attributed to their small size, large charge/ radius ratio and high electronegativity of the elements. In addition, the first member of group has only four valence orbitals (2s and 2p) available for bonding, whereas the second member of the groups have nine valence orbitals (3s, 3p, 3d). As a consequence of this, the maximum covalency of the first member of each group is 4 (e.g., boron can only form [BF4 ] − , whereas the other members of the groups can expand their valence shell to accommodate more than four pairs of electrons e.g., aluminium forms [AlF6 ]3−). Furthermore, the first member of p-block elements displays greater ability to form pπ – pπ multiple bonds to itself (e.g., C = C, C ≡ C, N = N, N ≡ Ν) and to other second period elements (e.g., C = O, C = N, C ≡ N, N = O) compared to subsequent members of the same group.
🙂
Ans : ( a, c )
🙂
Problem 3.9
Are the oxidation state and covalency of Al in [AlCl(H2O)5]2+ same ?
Solution
No. The oxidation state of Al is +3 and the covalency is 6.
🙂
Ans : ( a, c )
🙂
3.7.3 Periodic Trends and Chemical Reactivity
We have observed the periodic trends in certain fundamental properties such as atomic and ionic radii, ionization enthalpy, electron gain enthalpy and valence. We know by now that the periodicity is related to electronic configuration. That is, all chemical and physical properties are a manifestation of the electronic configuration of elements. We shall now try to explore relationships between these fundamental properties of elements with their chemical reactivity.
The atomic and ionic radii, as we know, generally decrease in a period from left to right. As a consequence, the ionization enthalpies generally increase (with some exceptions as outlined in section 3.7.1(a)) and electron gain enthalpies become more negative across a period. In other words, the ionization enthalpy of the extreme left element in a period is the least and the electron gain enthalpy of the element on the extreme right is the highest negative (note : noble gases having completely filled shells have rather positive electron gain enthalpy values). This results into high chemical reactivity at the two extremes and the lowest in the centre. Thus, the maximum chemical reactivity at the extreme left (among alkali metals) is exhibited by the loss of an electron leading to the formation of a cation and at the extreme right (among halogens) shown by the gain of an electron forming an anion. This property can be related with the reducing and oxidizing behaviour of the elements which you will learn later. However, here it can be directly related to the metallic and non-metallic character of elements. Thus, the metallic character of an element, which is highest at the extremely left decreases and the non-metallic character increases while moving from left to right across the period. The chemical reactivity of an element can be best shown by its reactions with oxygen and halogens. Here, we shall consider the reaction of the elements with oxygen only. Elements on two extremes of a period easily combine with oxygen to form oxides. The normal oxide formed by the element on extreme left is the most basic (e.g., Na2O), whereas that formed by the element on extreme right is the most acidic (e.g., Cl2O7). Oxides of elements in the centre are amphoteric (e.g., Al2O3, As2O3) or neutral (e.g., CO, NO, N2O). Amphoteric oxides behave as acidic with bases and as basic with acids, whereas neutral oxides have no acidic or basic properties.
🙂
Ans : ( b, d )
🙂
Problem 3.10
Show by a chemical reaction with water that Na2O is a basic oxide and Cl2O7 is an acidic oxide.
Solution
Na2O with water forms a strong base whereas Cl2O7 forms strong acid.
Na2O + H2O → 2NaOH Cl2O7 + H2O → 2HClO4
Their basic or acidic nature can be qualitatively tested with litmus paper.
Among transition metals (3d series), the change in atomic radii is much smaller as compared to those of representative elements across the period. The change in atomic radii is still smaller among inner-transition metals (4f series). The ionization enthalpies are intermediate between those of s- and p-blocks. As a consequence, they are less electropositive than group 1 and 2 metals.
In a group, the increase in atomic and ionic radii with increase in atomic number generally results in a gradual decrease in ionization enthalpies and a regular decrease (with exception in some third period elements as shown in section 3.7.1(d)) in electron gain enthalpies in the case of main group elements. Thus, the metallic character increases down the group and non-metallic character decreases. This trend can be related with their reducing and oxidizing property which you will learn later. In the case of transition elements, however, a reverse trend is observed. This can be explained in terms of atomic size and ionization enthalpy.
In this Unit, you have studied the development of the Periodic Law and the Periodic Table. Mendeleev’s Periodic Table was based on atomic masses. Modern Periodic Table arranges the elements in the order of their atomic numbers in seven horizontal rows (periods) and eighteen vertical columns (groups or families). Atomic numbers in a period are consecutive, whereas in a group they increase in a pattern. Elements of the same group have similar valence shell electronic configuration and, therefore, exhibit similar chemical properties. However, the elements of the same period have incrementally increasing number of electrons from left to right, and, therefore, have different valencies. Four types of elements can be recognized in the periodic table on the basis of their electronic configurations. These are s-block, p-block, d-block and f-block elements. Hydrogen with one electron in the 1s orbital occupies a unique position in the periodic table. Metals comprise more than seventy eight per cent of the known elements. Nonmetals,which are located at the top of the periodic table, are less than twenty in number. Elements which lie at the border line between metals and non-metals (e.g., Si, Ge, As) are called metalloids or semi-metals. Metallic character increases with increasing atomic number in a group whereas decreases from left to right in a period. The physical and chemical properties of elements vary periodically with their atomic numbers.
Periodic trends are observed in atomic sizes, ionization enthalpies, electron gain enthalpies, electronegativity and valence. The atomic radii decrease while going from left to right in a period and increase with atomic number in a group. Ionization enthalpies generally increase across a period and decrease down a group. Electronegativity also shows a similar trend. Electron gain enthalpies, in general, become more negative across a period and less negative down a group. There is some periodicity in valence, for example, among representative elements, the valence is either equal to the number of electrons in the outermost orbitals or eight minus this number. Chemical reactivity is hightest at the two extremes of a period and is lowest in the centre. The reactivity on the left extreme of a period is because of the ease of electron loss (or low ionization enthalpy). Highly reactive elements do not occur in nature in free state; they usually occur in the combined form. Oxides formed of the elements on the left are basic and of the elements on the right are acidic in nature. Oxides of elements in the centre are amphoteric or neutral.
3.1 What is the basic theme of organisation in the periodic table?
3.2 Which important property did Mendeleev use to classify the elements in his periodic table and did he stick to that?
3.3 What is the basic difference in approach between the Mendeleev’s Periodic Law and the Modern Periodic Law?
3.4 On the basis of quantum numbers, justify that the sixth period of the periodic table should have 32 elements.
3.5 In terms of period and group where would you locate the element with Z =114?
3.6 Write the atomic number of the element present in the third period and seventeenth group of the periodic table.
3.7 Which element do you think would have been named by (i) Lawrence Berkeley Laboratory (ii) Seaborg’s group?
3.8 Why do elements in the same group have similar physical and chemical properties?
3.9 What does atomic radius and ionic radius really mean to you?
3.10 How do atomic radius vary in a period and in a group? How do you explain the variation?
3.11 What do you understand by isoelectronic species? Name a species that will be isoelectronic with each of the following atoms or ions. (i) F– (ii) Ar (iii) Mg2+ (iv) Rb+
3.12 Consider the following species : N3–, O2–, F–, Na+, Mg2+ and Al3+ (a) What is common in them? (b) Arrange them in the order of increasing ionic radii.
3.13 Explain why cation are smaller and anions larger in radii than their parent atoms?
3.14 What is the significance of the terms – ‘isolated gaseous atom’ and ‘ground state’ while defining the ionization enthalpy and electron gain enthalpy? Hint : Requirements for comparison purposes.
3.15 Energy of an electron in the ground state of the hydrogen atom is -2.18×1018J. Calculate the ionization enthalpy of atomic hydrogen in terms of J mol-1 . Hint: Apply the idea of mole concept to derive the answer.
3.16 Among the second period elements the actual ionization enthalpies are in the order Li < B < Be < C < O < N < F < Ne. Explain why (i) Be has higher Δi H than B (ii) O has lower Δi H than N and F?
3.17 How would you explain the fact that the first ionization enthalpy of sodium is lower than that of magnesium but its second ionization enthalpy is higher than that of magnesium?
3.18 What are the various factors due to which the ionization enthalpy of the main group elements tends to decrease down a group?
3.19 The first ionization enthalpy values (in kJ mol-1) of group 13 elements are : B Al Ga In Tl 801 577 579 558 589 How would you explain this deviation from the general trend ?
3.20 Which of the following pairs of elements would have a more negative electron gain enthalpy? (i) O or F (ii) F or Cl
3.21 Would you expect the second electron gain enthalpy of O as positive, more negative or less negative than the first? Justify your answer.
3.22 What is the basic difference between the terms electron gain enthalpy and electronegativity?
3.23 How would you react to the statement that the electronegativity of N on Pauling scale is 3.0 in all the nitrogen compounds?
3.24 Describe the theory associated with the radius of an atom as it (a) gains an electron (b) loses an electron
3.25 Would you expect the first ionization enthalpies for two isotopes of the same element to be the same or different? Justify your answer.
3.26 What are the major differences between metals and non-metals?
3.27 Use the periodic table to answer the following questions. (a) Identify an element with five electrons in the outer subshell. (b) Identify an element that would tend to lose two electrons. (c) Identify an element that would tend to gain two electrons. (d) Identify the group having metal, non-metal, liquid as well as gas at the room temperature.
3.28 The increasing order of reactivity among group 1 elements is Li < Na < K < Rb CI > Br > I. Explain.
3.29 Write the general outer electronic configuration of s-, p-, d- and f- block elements.
3.30 Assign the position of the element having outer electronic configuration (i) ns2np4 for n=3 (ii) (n-1)d2ns2 for n=4, and (iii) (n-2) f7 (n-1)d1ns2 for n=6, in the periodic table.
3.31 The first (ΔiH1) and the second (ΔiH2) ionization enthalpies (in kJ mol-1) and the (ΔegH) electron gain enthalpy (in kJ mol-i) of a few elements are given below:
Elements ΔH11 ΔH2 ΔegH
I 5 20 7300 -60
II 419 3051 -48
III 1681 3374 -328
IV 1008 1846 -295
V 2372 5251 +48
VI 738 1451 -40
Which of the above elements is likely to be :
(a) the least reactive element.
(b) the most reactive metal.
(c) the most reactive non-metal.
(d) the least reactive non-metal.
(e) the metal which can form a stable binary halide of the formula MX2(X=halogen).
(f) the metal which can form a predominantly stable covalent halide of the formula MX (X=halogen)?
3.32 Predict the formulas of the stable binary compounds that would be formed by the combination of the following pairs of elements.
(a) Lithium and oxygen (b) Magnesium and nitrogen (c) Aluminium and iodine (d) Silicon and oxygen (e) Phosphorus and fluorine (f) Element 71 and fluorine
3.33 In the modern periodic table, the period indicates the value of :
(a) atomic number (b) atomic mass (c) principal quantum number (d) azimuthal quantum number.
3.34 Which of the following statements related to the modern periodic table is incorrect? (a) The p-block has 6 columns, because a maximum of 6 electrons can occupy all the orbitals in a p-shell. (b) The d-block has 8 columns, because a maximum of 8 electrons can occupy all the orbitals in a d-subshell. (c) Each block contains a number of columns equal to the number of electrons that can occupy that subshell. (d) The block indicates value of azimuthal quantum number (l) for the last subshell that received electrons in building up the electronic configuration.
3.35 Anything that influences the valence electrons will affect the chemistry of the element. Which one of the following factors does not affect the valence shell?
(a) Valence principal quantum number (n)
(b) Nuclear charge (Z )
(c) Nuclear mass
(d) Number of core electrons .
3.36 The size of isoelectronic species – F–, Ne and Na+ is affected by
(a) nuclear charge (Z )
(b) valence principal quantum number (n)
(c) electron-electron interaction in the outer orbitals
(d) none of the factors because their size is the same.
3.37 Which one of the following statements is incorrect in relation to ionization enthalpy?
(a) Ionization enthalpy increases for each successive electron.
(b) The greatest increase in ionization enthalpy is experienced on removal of electron from core noble gas configuration.
(c) End of valence electrons is marked by a big jump in ionization enthalpy.
(d) Removal of electron from orbitals bearing lower n value is easier than from orbital having higher n value.
3.38 Considering the elements B, Al, Mg, and K, the correct order of their metallic character is :
(a) B > Al > Mg > K (b) Al > Mg > B > K (c) Mg > Al > K > B (d) K > Mg > Al > B
🙂
Ans :
i ( b ) ii ( c ) iii ( a )
🙂
3.39 Considering the elements B, C, N, F, and Si, the correct order of their non-metallic character is :
(a) B > C > Si > N > F (b) Si > C > B > N > F (c) F > N > C > B > Si (d) F > N > C > Si > B
3.40 Considering the elements F, Cl, O and N, the correct order of their chemical reactivity in terms of oxidizing property is :
(a) F > Cl > O > N (b) F > O > Cl > N (c) Cl > F > O > N (d) O > F > N > Cl
I. Multiple Choice Questions (Type-I)
1. Consider the isoelectronic species, Na+, Mg2+, F– and O2-. The correct order of increasing length of their radii is _________.
(i) F– 2– < Mg2+ < Na+
(ii) Mg2+ < Na+ < F– < O2–
(iii) O2– < F– < Na+ < Mg2+
(iv) O2– < F– < Mg2+ < Na+
2. Which of the following is not an actinoid?
(i) Curium (Z = 96)
(ii) Californium (Z = 98)
(iii) Uranium (Z = 92)
(iv) Terbium (Z = 65)
3. The order of screening effect of electrons of s, p, d and f orbitals of a given shell of an atom on its outer shell electrons is:
(i) s > p > d > f
(ii) f > d > p > s
(iii) p < d < s > f
(iv) f > p > s > d
4. The first ionisation enthalpies of Na, Mg, Al and Si are in the order:
(i) Na < Mg > Al < Si (ii) Na > Mg > Al > Si
(iii) Na < Mg < Al < Si (iv) Na > Mg > Al < Si
5. The electronic configuration of gadolinium (Atomic number 64) is
(i) [Xe] 4f3 5d5 6s2
(ii) [Xe] 4f7 5d2 6s1
(iii) [Xe] 4f7 5d1 6s2
(iv) [Xe] 4f8 5d6 6s2
6. The statement that is not correct for periodic classification of elements is:
(i) The properties of elements are periodic function of their atomic numbers.
(ii) Non metallic elements are less in number than metallic elements.
(iii) For transition elements, the 3d-orbitals are filled with electrons after 3p-orbitals and before 4s-orbitals.
(iv) The first ionisation enthalpies of elements generally increase with increase in atomic number as we go along a period.
7. Among halogens, the correct order of amount of energy released in electron gain (electron gain enthalpy) is:
(i) F > Cl > Br > I
(ii) F < Cl < Br < I
(iii) F < Cl > Br > I
(iv) F < Cl < Br < I 8. The period number in the long form of the periodic table is equal to (i) magnetic quantum number of any element of the period. (ii) atomic number of any element of the period. (iii) maximum Principal quantum number of any element of the period. (iv) maximum Azimuthal quantum number of any element of the period. 9. The elements in which electrons are progressively filled in 4f-orbital are called (i) actinoids (ii) transition elements (iii) lanthanoids (iv) halogens 10. Which of the following is the correct order of size of the given species: (i) I > I– > I+
(ii) I+ > I– > I
(iii) I > I+ > I –
(iv) I– > I > I+
11. The formation of the oxide ion, O2– (g), from oxygen atom requires first an exothermic and then an endothermic step as shown below:
O(g) + e– → O–(g) ; Δ Hθ = –141 kJ mol–1
O–(g) + e– → O2– (g); Δ Hθ = +780 kJ mol–1
Thus process of formation of O2– in gas phase is unfavourable even though O2– is isoelectronic with neon. It is due to the fact that,
(i) oxygen is more electronegative.
(ii) addition of electron in oxygen results in larger size of the ion.
(iii) electron repulsion outweighs the stability gained by achieving noble gas configuration.
(iv) O– ion has comparatively smaller size than oxygen atom.
12. Comprehension given below is followed by some multiple choice questions. Each question has one correct option. Choose the correct option.
In the modern periodic table, elements are arranged in order of increasing atomic numbers which is related to the electronic configuration. Depending upon the type of orbitals receiving the last electron, the elements in the periodic table have been divided into four blocks, viz, s, p, d and f. The modern periodic table consists of 7 periods and 18 groups. Each period begins with the filling of a new energy shell. In accordance with the Arfbau principle, the seven periods (1 to 7) have 2, 8, 8, 18, 18, 32 and 32 elements respectively. The seventh period is still incomplete. To avoid the periodic table being too long, the two series of f-block elements, called lanthanoids and actinoids are placed at the bottom of the main body of the periodic table.
(a) The element with atomic number 57 belongs to
(i) s-block
(ii) p-block
(iii) d-block
(iv) f-block
(b) The last element of the p-block in 6th period is represented by the outermost electronic configuration.
(i) 7s2 7p6
(ii) 5f14 6d10 7s2 7p0
(iii) 4f14 5d10 6s2 6p6
(iv) 4f14 5d10 6s2 6p4
(c) Which of the elements whose atomic numbers are given below, cannot be accommodated in the present set up of the long form of the periodic table?
(i) 107
(ii) 118
(iii) 126
(iv) 102
(d) The electronic configuration of the element which is just above the element with atomic number 43 in the same group is ________.
(i) 1s2 2s2 2p6 3s2 3p6 3d5 4s2
(ii) 1s2 2s2 2p6 3s2 3p6 3d5 4s3 4p6
(iii) 1s2 2s2 2p6 3s2 3p6 3d6 4s2
(iv) 1s2 2s2 2p6 3s2 3p6 3d7 4s2
(e) The elements with atomic numbers 35, 53 and 85 are all ________.
(i) noble gases
(ii) halogens
(iii) heavy metals
(iv) light metals
13. Electronic configurations of four elements A, B, C and D are given below :
(A) 1s2 2s2 2p6 (B) 1s2 2s2 2p4
(C) 1s2 2s2 2p6 3s1 (D) 1s2 2s2 2p5
Which of the following is the correct order of increasing tendency to gain electron :
(i) A < C < B < D
(ii) A < B < C < D
(iii) D < B < C < A
(iv) D < A < B < C
II. Multiple Choice Questions (Type-II)
In the following questions two or more options may be correct.
14. Which of the following elements can show covalency greater than 4?
(i) Be
(ii) P
(iii) S
(iv) B
15. Those elements impart colour to the flame on heating in it, the atoms of which require low energy for the ionisation (i.e., absorb energy in the visible region of spectrum). The elements of which of the following groups will impart colour to the flame?
(i) 2
(ii) 13
(iii) 1
(iv) 17
16. Which of the following sequences contain atomic numbers of only representative elements?
(i) 3, 33, 53, 87
(ii) 2, 10, 22, 36
(iii) 7, 17, 25, 37, 48
(iv) 9, 35, 51, 88
17. Which of the following elements will gain one electron more readily in comparison to other elements of their group?
(i) S (g)
(ii) Na (g)
(iii) O (g)
(iv) Cl (g)
18. Which of the following statements are correct?
(i) Helium has the highest first ionisation enthalpy in the periodic table.
(ii) Chlorine has less negative electron gain enthalpy than fluorine.
(iii) Mercury and bromine are liquids at room temperature.
(iv) In any period, atomic radius of alkali metal is the highest.
19. Which of the following sets contain only isoelectronic ions?
(i) Zn2+, Ca2+, Ga3+, Al3+
(ii) K+, Ca2+, Sc3+, Cl–
(iii) P3–, S2–, Cl–, K+
(iv) Ti4+, Ar, Cr3+, V5+
20. In which of the following options order of arrangement does not agree with the variation of property indicated against it?
(i) Al3+ < Mg2+ < Na+ < F– (increasing ionic size)
(ii) B < C < N < O (increasing first ionisation enthalpy)
(iii) I < Br < Cl < F (increasing electron gain enthalpy)
(iv) Li < Na < K < Rb (increasing metallic radius)
21. Which of the following have no unit?
(i) Electronegativity
(ii) Electron gain enthalpy
(iii) Ionisation enthalpy
(iv) Metallic character
22. Ionic radii vary in
(i) inverse proportion to the effective nuclear charge.
(ii) inverse proportion to the square of effective nuclear charge.
(iii) direct proportion to the screening effect.
(iv) direct proportion to the square of screening effect.
23. An element belongs to 3rd period and group-13 of the periodic table. Which of the following properties will be shown by the element?
(i) Good conductor of electricity
(ii) Liquid, metallic
(iii) Solid, metallic
(iv) Solid, non metallic
III. Short Answer Type
24. Explain why the electron gain enthalpy of fluorine is less negative than that of chlorine.
25. All transition elements are d-block elements, but all d-block elements are not transition elements. Explain.
26. Identify the group and valency of the element having atomic number 119. Also predict the outermost electronic configuration and write the general formula of its oxide.
27. Ionisation enthalpies of elements of second period are given below :
Ionisation enthalpy/ k cal mol–1 : 520, 899, 801, 1086, 1402, 1314,1681, 2080.
Match the correct enthalpy with the elements and complete the graph given in Fig. 3.1. Also write symbols of elements with their atomic number.
🙂
🙂
28. Among the elements B, Al,C and Si,
(i) which element has the highest first ionisation enthalpy?
(ii) which element has the most metallic character?
Justify your answer in each case.
29. Write four characteristic properties of p-block elements.
30. Choose the correct order of atomic radii of fluorine and neon (in pm) out of the options given below and justify your answer.
(i) 72, 160
(ii) 160, 160
(iii) 72, 72
(iv) 160, 72
31. Illustrate by taking examples of transition elements and non-transition elements that oxidation states of elements are largely based on electronic configuration.
32. Nitrogen has positive electron gain enthalpy whereas oxygen has negative. However, oxygen has lower ionisation enthalpy than nitrogen. Explain.
33. First member of each group of representative elements (i.e., s and p-block elements) shows anomalous behaviour. Illustrate with two examples.
34. p-Block elements form acidic, basic and amphoteric oxides. Explain each property by giving two examples and also write the reactions of these oxides with water.
35. How would you explain the fact that first ionisation enthalpy of sodium is lower than that of magnesium but its second ionisation enthalpy is higher than that of magnesium?
36. What do you understand by exothermic reaction and endothermic reaction? Give one example of each type.
37. Arrange the elements N, P, O and S in the order of-
(i) increasing first ionisation enthalpy.
(ii) increasing non metallic character.
Give reason for the arrangement assigned.
38. Explain the deviation in ionisation enthalpy of some elements from the general trend by using Fig. 3.2.
🙂
🙂
39. Explain the following:
(a) Electronegativity of elements increase on moving from left to right in the periodic table.
(b) Ionisation enthalpy decrease in a group from top to bottom?
40. How does the metallic and non metallic character vary on moving from left to right in a period?
41. The radius of Na+ cation is less than that of Na atom. Give reason.
42. Among alkali metals which element do you expect to be least electronegative and why?
IV. Matching Type
43. Match the correct atomic radius with the element.
🙂
🙂
44. Match the correct ionisation enthalpies and electron gain enthalpies of the following elements.
🙂
45. Electronic configuration of some elements is given in Column I and their electron gain enthalpies are given in Column II. Match the electronic configuration with electron gain enthalpy.
🙂
V. Assertion and Reason Type
In the following questions a statement of Assertion (A) followed by a statement of reason (R) is given. Choose the correct option out of the choices given below each question.
46. Assertion (A) : Generally, ionisation enthalpy increases from left to right in a period.
Reason (R) : When successive electrons are added to the orbitals in the same principal quantum level, the shielding effect of inner core of electrons does not increase very much to compensate for the increased attraction of the electron to the nucleus.
(i) Assertion is correct statement and reason is wrong statement.
(ii) Assertion and reason both are correct statements and reason is correct explanation of assertion.
(iii) Assertion and reason both are wrong statements.
(iv) Assertion is wrong statement and reason is correct statement.
47. Assertion (A) : Boron has a smaller first ionisation enthalpy than beryllium.
Reason (R) : The penetration of a 2s electron to the nucleus is more than the 2p electron hence 2p electron is more shielded by the inner core of electrons than the 2s electrons.
(i) Assertion and reason both are correct statements but reason is not correct explanation for assertion.
(ii) Assertion is correct statement but reason is wrong statement.
(iii) Assertion and reason both are correct statements and reason is correct explanation for assertion.
(iv) Assertion and reason both are wrong statements.
48. Assertion (A) : Electron gain enthalpy becomes less negative as we go down a group.
Reason (R) : Size of the atom increases on going down the group and the added electron would be farther from the nucleus.
(i) Assertion and reason both are correct statements but reason is not correct explanation for assertion.
(ii) Assertion and reason both are correct statements and reason is correct explanation for assertion.
(iii) Assertion and reason both are wrong statements.
(iv) Assertion is wrong statement but reason is correct statement.
VI. Long Answer Type
49. Discuss the factors affecting electron gain enthalpy and the trend in its variation in the periodic table.
50. Define ionisation enthalpy. Discuss the factors affecting ionisation enthalpy of the elements and its trends in the periodic table.
51. Justify the given statement with suitable examples— “the Properties of the elements are a periodic function of their atomic numbers”.
52. Write down the outermost electronic configuration of alkali metals. How will you justify their placement in group 1 of the periodic table?
53. Write the drawbacks in Mendeleev’s periodic table that led to its modification.
54. In what manner is the long form of periodic table better than Mendeleev’s periodic table? Explain with examples.
55. Discuss and compare the trend in ionisation enthalpy of the elements of group1 with those of group17 elements.
ANSWERS
I. Multiple Choice Questions (Type-I)
1. (ii) 2. (iv) 3. (i) 4. (i) 5. (iii) 6. (iii) 7. (iii) 8. (iii) 9. (iii) 10. (iv) 11. (iii) 12.(a) (iii), (b) (iii), (c) (iii), (d) (i), (e) (ii) 13. (i)
II. Multiple Choice Questions (Type-II)
14. (ii), (iii) 15. (i), (iii) 16. (i), (iv) 17. (i), (iv) 18. (i), (iii), (iv) 19. (ii), (iii) 20. (ii), (iii) 21. (i), (iv) 22. (i), (iii) 23. (i), (iii)
III. Short Answer Type
24. The added electron in fluorine goes to second quantum level. Due to small size of fluorine it experiences repulsion from other electrons much more in comparison to that in chlorine because in chlorine, the electron is added to 3rd quantum level in which larger space is available for movement.
26. Group : 1, Valency : 1
Outermost electronic configuration = 8s1
Formula of Oxide = M2O
27. Compare your plot with the plot given in the textbook.
28. (i) Carbon
(ii) Aluminium
30. (i)
🙂
Ans :
i ( b ) ii ( a ) iii ( c )
🙂
32. The outermost electronic configuraton of nitrogen (2s2 2px1 2py1 2pz1 ) is very stable because p-orbital is half filled. Addition of extra electron to any of the 2p orbital requires energy.
Oxygen has 4 electrons in 2p orbitals and acquires stable configuration i.e., 2p3 configuration after removing one electron.
35. After removing 1 electron from the sodium atom the ion formed acquires the configuration of inert gas, neon. The second electron is removed from
one of the 2p-orbitals which are completely filled i.e., have a total of 6 electrons and are closer to the nucleus.
37. (i) S < P < N < O
(ii) P < S < N < O
39. (a) Decrease in size of atom and increase in nuclear charge.
(b) Increase in atomic size.
40. Metallic character decreases and non metallic character increases in moving from left to right in a period. It is due to increase in ionisation enthalpy
and electron gain enthalpy.
41. Decrease of one shell.
42. Electronegativity decreases in a group from top to bottom. Thus, caesium is the least electronegative element.
🙂
Ans : i ( b ) ii ( a ) iii ( c )
🙂
IV. Matching Type
43. Be = 111, O = 66, C = 77, B = 88, N = 74.
44. Most reactive non metal = B, Most reactive metal = A, Least reactive element = D, Metal forming binary halide = C
45. (i) → (D); (ii) → (A) (iii) → (B) (iv) → (C)
V. Assertion and Reason Type
46. (ii)
47. (iii)
48. (iv)
xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx
In youtube search for Zookeeper Dezrina you will get most videos. I say most because I do not upload all videos that I make. I have many more videos which are not in the net.
https://zookeepersblog.wordpress.com/iit-jee-capacitance/
https://zookeepersblog.wordpress.com/iit-jee-3d-geometry-solutions/
https://zookeepersblog.wordpress.com/iit-jee-algebra/
🙂
Ans : i ( b ) ii ( c ) iii ( d )
🙂
https://zookeepersblog.wordpress.com/iit-jee-area-problems/
https://zookeepersblog.wordpress.com/iit-jee-binomial-theorem/
https://zookeepersblog.wordpress.com/iit-jee-calculus/
https://zookeepersblog.wordpress.com/iit-jee-optics/
https://zookeepersblog.wordpress.com/iit-jee-co-ordinate-geometry/
https://zookeepersblog.wordpress.com/iit-jee-complex-number/
🙂
Ans : ( c )
🙂
https://zookeepersblog.wordpress.com/iit-jee-current-electricity-circuits/
https://zookeepersblog.wordpress.com/iit-jee-determinant-and-matrices/
🙂
Ans : i ( a ) ii ( d ) iii ( b )
🙂
https://zookeepersblog.wordpress.com/iit-jee-differentiability/
https://zookeepersblog.wordpress.com/iit-jee-electromagnetics/
https://zookeepersblog.wordpress.com/iit-jee-electrostatics/
xxxxxxxxxxxxxxxxxxxxxx
Ans : ( a )
Fluorine being most electronegative always exhibits -1 oxidation state
xxxxxxxxxxxxxxxxxxxxxx
The following Videos are available for you ( As of Now ). These explain tricky Physics and Mathematics Numericals.
🙂
Ans : ( c )
🙂
Eventually I will try to give Videos for full course here for you.
These covers PU ( Pre University courses, school / college ) courses, IIT JEE, AIEEE ( All India Engineering Entrance Examination ) , CET ( Combined Engineering Test ), AIPMT ( All India Pre Medical Test ), ISc ( Intermediate Science / Indian School Certificate Exam ), CBSE ( Central Board Secondary Exam ), Roorkey Joint Entrance Test Questions ( Discontinued since 2002 ), APhO ( Asian Physics Olympiad ), IPhO ( International Physics Olympiad ), IMO ( International Mathematics Olympiad ) , NSEP ( National Standard Exam in Physics ), RMO ( Regional Math Olympiad , India ), INMO ( Indian National Maths Olympiad ), Irodov Solutions, Prof. H C Verma ( Concepts of Physics ) Solutions etc.
🙂
Ans : ( c )
🙂
( You can see the history of Indian Participation in various Olympiads at -> https://zookeepersblog.wordpress.com/indian-participation-in-ipho-icho-ibo-and-astronomy-olympiad/ )
[ In each of these videos there is at-least 1 or more errors. Please tell me about those ]
In youtube if you search as ” Dezrina ” or ” Zookeeper Physics ” you should get to see all the Uploaded videos. Though we have many more study videos.
Thanks and Regards
Zookeeper ;-D Subhashish Chattopadhyay
🙂
Ans : ( a )
🙂
[ I suggest you see the videos starting with 1- first then starting with 2- ….. in that sequence. ]
[ Tell your friends about this link if you liked the videos ]
In case of doubts or suggestions, Please send me email at mokshya@gmail.com
In youtube you can search for Dezrina as these have been uploaded with this login-id. or search for ” Zookeeper IIT JEE Physics “
Answers to -> Frequently Asked Questions ( FAQ ) [ commonly asked intelligent Questions 🙂 ]
🙂
Ans : ( d )
Students should know that Cl-Cl bond is strongest
🙂
1 ) How do I prepare for IIT ?
Ans : – See the videos made by me ( in youtube search as Dezrina or “ Zookeeper Physics “ – will see all Uploaded ones. Though we have many more which have not been uploaded ). While watching the videos, take notes and try to solve the problems yourself by pausing the video. Tell me if any calculation is wrong. See the videos with 1- first then 2- and so on. Write to IAPT Kothrud, Pune office to buy ( 150 Rs approx ) the book with previous papers of NSEP ( National Standard Exam in Physics – The 1st level ), INPhO ( Indian National Physics Olympiad – 2nd level ). Prepare with these and see how much you are scoring. You can guess your ALL INDIA rank easily from NSEP, and INPhO rank. Since 1998 the IIT JEE toppers have been mostly representing India in IPhO.
🙂
Ans : ( d )
🙂
2 ) Which codec and Player do I use to see the videos ?
Ans : – You can use GOM Player, or VLC Player. You have to have good speakers with filters or good earphones with filters. We have checked mostly it is OKwith these. ( If you are depending only on your embedded speakers of computer /screen / keyboard then there may be extra distortions. As these speakers are
often not of good Quality. Also install latest KL Codecs ) In any case reduce the volume see the board, imagine sitting in the last bench and solving the
problems of your own. See if your solution differs anywhere with the scribbles on the board.
Ans : ( b )
🙂
3 ) Why are you giving these ( high Quality ) lecture for free ?
Ans : Well there are lot of good things free in this world. Linux, My-SQL, Open-Office ….. Go to sourceforge and get thousands of high quality software free along with source code. Yes all officially free …. Why do you think Richard Stallman, Zimmerman, ….. etc are considered Guru philosophers ? In Punjab and Gurudwaras worldwide there are so many Langars where you get better food than Restaurants. … why ? Why do you have Dharmasalas and subsidized rest rooms near hospitals / Famous Temples / various places ? in Iftar party anyone can eat for free … why ? I am teaching for 20 years now and observed most students can do much better if they have the self motivation to solve and practice. Cheap books are available in second hand bookstalls, where you get thousands of Numericals to solve ….. but most students will like to blow their time going and coming for tuition, travel time …. TV for hours and hours watching cricket / Tennis games, playing computer games …. My free lectures are not going to make much difference in spending of unnecessary money for coaching ….. I know very well , how much people enjoy .. , spending unnecessarily !! Do you know that there are NO poor / needy students in Bangalore. Sometime back I had tried to teach for IIT JEE FREE. Discussed with a few NGOs and social service guys. Arranged rooms but got only 1 student. We had informed many people in many ways to inform students …. We did not get students who are ready to learn for free. So I am sure these lectures are NOT FREE. If anyone learns from these, s/he changes and that’s the gain / benefit. This change ( due to learning ) is very costly …. Most do not want to learn ………..
In youtube search for Zookeeper Dezrina you will get most videos. I say most because I do not upload all videos that I make. I have many more videos which are not in the net.
🙂
Ans : ( a )
The stability of hydrides in group 16 decreases down the group
🙂
Ans : i ( b ) ii ( d ) iii ( b )
🙂
4 ) How can I get all your lectures ?
Ans : – Apart from my lectures there are approx 700 GB of PCM ( Phy, Chem, Math ) lectures. It takes approx 3 years of continuous download from scattered
sources. I have ( 20,000 )Thousands of these. You can take ALL of them from me in an external 1 TB hard disk, instead of spending so much money and time
again for downloading. These cover ( by Various Professors ) everything of Chemistry, Physics, Maths… Lot of this is from outside India … as foreigners
have much wider heart than Indians ( as most of GNU / open source software have been developed by Non-Indians ). I observed the gaps in these videos, and
thus I am solving IIT, APhO, Roorkey, IPhO Numericals. Videos made by me along with these videos gives a complete preparation.
Send me a mail at mokshya@gmail.com to contact me.
search for videos in http://www.skmclasses.weebly.com
You will get most videos. I say most because I do not upload all videos that I make. I have many more videos which are not in the net.
🙂
Send me a mail at mokshya@gmail.com to contact me.
In youtube search for Zookeeper Dezrina you will get most videos. I say most because I do not upload all videos that I make. I have many more videos which are not in the net.
🙂
Ans : ( b )
🙂
5 ) How do you get benefited out of this ?
Ans :- If anyone learns we all will have better people in this world. I will have better “ YOU “.
🙂
Ans : ( b )
🙂
6 ) Why do you call yourself a Zookeeper ?
Ans :- This is very nicely explained at
https://zookeepersblog.wordpress.com/z00keeper-why-do-i-call-myself-a-zoookeeper/
🙂
Ans : ( b )
🙂
7 ) Where do you stay ?
Ans :- Presently I am in Bangalore.
🙂
Ans : ( c )
Because of Hydrogen bonding melting point of NH3 is greater than PH3
🙂
8 ) If I need videos in a few topics can you make them for me ?
Ans :- Yes. You have to discuss the urgency with me. If I am convinced I will surely make these quickly for you and give you and ALL. I teach both Maths and Physics. So anything in these 2 subjects are welcome.
🙂
Ans : ( a )
Boiling point decreases Si to Pb
🙂
9 ) Why did you write an article saying there are No Poor students ?
Ans :- There are lots of NGOs and others working for rural / poor children education at lower classes. While very less effort is on for std 9 till 12. Also see the answer in question number ( 3 ) above. In last 20 years of teaching I never met a Poor child who was seriously interested in ( higher ) studies. As I have a mind / thinking of a ” Physicist “, I go by ” Experimental Observation “.
🙂
Ans : ( c )
Because of catenation of Carbon its melting point is higher than Silicon
🙂
It is not about what is being said about poor in media / TV etc, or ” what it should be ” ( ? ) …. It is about what I see happening. Also to add ( confuse ? you more )…. You must be knowing that in several states over many years now girl students have better ( by marks as well as by pass percentage ) result in std 10 / Board Exams….. well but NEVER a girl student came FIRST in IIT JEE … why ? [ The best rank by a Girl student is mostly in 2 digits, very rarely in single digit ] ????? So ????
🙂
Ans : ( d )
🙂
10 ) How much do I have to study to make it to IIT ?
Ans :- My experience of Teaching for IIT JEE since last 20 years, tells me, Total 200 hours per subject ( PCM ) is sufficient. If you see my Maths and Physics videos, each subject is more than 200 hours. So if someone sees all the videos diligently, takes notes and remembers, …… Done.
🙂
Ans : ( c )
🙂
11 ) What is EAMCET ?
Ans :- Engineering Agriculture and Medicine Common Entrance Test is conducted by JNT University Hyderabad on behalf of APSCHE. This examination is the gateway for entry into various professional courses offered in Government/Private Colleges in Andhra Pradesh.
🙂
Ans : ( d )
🙂
12 ) In your videos are you covering other Exams apart from IIT ?
Ans : – Yes. See many videos made by solving problems of MPPET, Rajasthan / J&K CET, UPSEAT ( UPES Engineering Aptitude Test ), MHCET, BCECE ( Bihar Combined Entrance Competitive Examination Board ), WB JEE etc
🙂
Ans : ( b )
🙂
13 ) What is SCRA ?
Ans : – Special Class Railway Apprentice (SCRA) exam is conducted by Union Public Service Commission (UPSC) board, for about 10 seats.That translates into an astonishing ratio of 1 selection per 10,000 applicants. The SCRA scheme was started in 1927 by the British, to select a handful of most intelligent Indians to assist them in their Railway Operations, after training at their Railway’s largest workshop, i.e. Jamalpur Workshop, and for one year in United Kingdom. The selected candidates were required to appear in the Mechanical Engineering Degree Examination held by Engineering Council (London).
Thanks for your time. To become my friend in google+
( search me as mokshya@gmail.com and send friend request )
Read
http://edge.org/responses/what-scientific-concept-would-improve-everybodys-cognitive-toolkit
The following video is a must see for full CO2 cycle, plates of Earth, Geological activities, stability of weather
http://www.youtube.com/watch?v=Qtv_JD_I5X8
🙂
Ans :
i ( a ) ii ( a ) iii ( d )
🙂
–
Article in Nature says CO2 increase is good for the trees http://thegwpf.org/science-news/6086-co2-is-greening-the-planet-savannahs-soon-to-be-covered-by-forests.html
–
Ice cap variations, Temperature and humidity fluctuations nicely explained
http://www.youtube.com/watch?v=pz-w4NWRObw
http://climaterealists.com/index.php?id=9752
BBC documentary Crescent and Cross shows the 1000 years of fight between Christians and Muslims. Millions have been killed in the name of Religion. To decided whose GOD is better, and which GOD to follow. The fight continues. http://www.youtube.com/watch?v=zqK-RuntywY
🙂
Ans : ( b ) Boron has very low electrical conductivity
🙂
The Virus of Faith
http://www.youtube.com/watch?v=scarHc8RA0g
🙂
Ans : ( a, b, c, d )
🙂
The God delusion
http://www.youtube.com/watch?v=LVr9bJ8Sctk
cassiopeia facts about evolution
http://www.youtube.com/watch?v=K7tQIB4UdiY
Intermediate Fossil records shown and explained nicely Fossils, Genes, and Embryos
http://www.youtube.com/watch?v=fdpMrE7BdHQ
🙂
Ans : ( c )
🙂
The Rise Of Narcissism In Women http://www.youtube.com/watch?v=wZHKCbHGlS0
13 type of women whom you should never court
http://timesofindia.indiatimes.com/life-style/relationships/man-woman/13-Women-you-should-never-court/articleshow/14637014.cms
Media teaching Misandry in India www.youtube.com/watch?v=-M2txSbOPIo
🙂
Ans : ( a )
🙂
Summary of problems with women
http://problemwithwomentoday.blogspot.in/2009/12/problem-with-women-today-what-in-hell.html
🙂
Ans : ( a, b, c )
🙂
Eyeopener men ? women only exists
www.youtube.com/watch?v=6ZAuqkqxk9A
Most unfortunate for men
http://www.youtube.com/watch?v=73fGqUwmOPg
🙂
Ans : ( a )
🙂
Each of you is an Activist in some way or other. You are trying to propagate those thoughts, ideas that you feel concerned / excited about.
Did you analyze your effectiveness ?
http://www.youtube.com/watch?v=61qn7S9NCOs
Culturomics can help you
😀
🙂
Ans : ( a, b, d )
The first ionization energy tends to increase along the period
🙂
Ans : ( a )
🙂
see how biased women are. Experimental proof. Women are happy when they see another woman is beating a man ( see how women misbehave with men )
www.youtube.com/watch?v=LlFAd4YdQks
🙂
Ans : ( d )
🙂
see detailed statistics at
http://www.youtube.com/watch?v=5lHmCN3MBMI
An eye opener in Misandry
http://www.youtube.com/watch?v=YiTaDS_X6CU
🙂
Amphoteric Oxide
Ans : ( b )
🙂
My sincere advice would be to be EXTREMELY careful ( and preferably away ) of girls. As girls age; statistically certain behavior in them has been observed. Most Male can NOT manage those behaviors… Domestic violence, divorce etc are rising very fast. Almost in all cases boys / males are HUGE loosers. Be extremely choosy ( and think from several angles ) before even talking to a girl.
https://zookeepersblog.wordpress.com/save-the-male/
How women manipulate men
http://www.angryharry.com/esWomenManipulateMen.htm
🙂
Which is Acidic oxide
Ans : ( a )
🙂
Gender Biased Laws in India
https://zookeepersblog.wordpress.com/biased-laws/
Violence against Men
http://www.youtube.com/watch?v=MLS2E-rRynE
Only men are victimised
http://www.youtube.com/watch?v=4JA4EPRbWhQ
🙂
Ans : ( b )
🙂
Men are BETTER than women
http://www.menarebetterthanwomen.com/
see
http://www.youtube.com/watch?&v=T0xoKiH8JJM#!
🙂
Ans : ( all a, b, c, and d ) are correct
🙂
Male Psychology
http://www.youtube.com/watch?v=uwxgavf2xWE
Women are more violent than men
http://www.independent.co.uk/news/uk/home-news/women-are-more-violent-says-study-622388.html
🙂
Ans : ( b )
🙂
Misandry in Media
http://www.youtube.com/watch?v=j7U0r7vIrgM
In the year 2010, 168 men ended their lives everyday ( on average ). More husbands committed suicide than wives.
http://www.rediff.com/news/report/ncrb-stats-show-more-married-men-committing-suicide/20111028.htm
🙂
Ans : ( d ) Mg, and Ca are obtained by electrolytic reduction
🙂
It is EXTREMELY unfortunate that media projects men as fools, women as superiors, Husbands as servants, and replaceable morons. In ad after ad worldwide from so many companies, similar msg to disintegrate the world is being bombarded. It is highly unacceptable misandry
http://www.youtube.com/watch?v=oq14WHkFq30
🙂
Ans : ( a )
🙂
It is NOT at all funny that media shows violence against MEN. Some advertisers are trying to create a new ” Socially acceptable culture ” of slapping Men ( by modern city women ). We ( all men ) take objection to these advertisements. We oppose this Misandry bad culture. Please share to increase awareness against Men bashing
http://www.youtube.com/watch?v=D8ecN2rh0uU
🙂
Ans : ( b )
The solubility of Sulphates and Carbonates decreases down the group
🙂
Ans : ( a )
🙂
Think what are you doing … why are you doing ? http://www.youtube.com/watch?v=qp0HIF3SfI4
Every Man must know this … http://www.youtube.com/watch?v=cIFmQHJEG1M
Manginas, White Knights, & Other Chivalrous Dogs http://www.youtube.com/watch?v=oXQDtBT70B8
!
Ans : ( d )
The solubility of hydroxides increases down the group
!
: ****__********__***
…….. (””(`-“’´´-´)””)
……….)…..–…….–….(
………/…..(6…_…6)….\
………\……..(..0..)….;../
……__.`.-._..’=’…_.-.`.__
…./……’###.,.–.,.###.’…\
….\__))####’#’###(((__/
……##### u r #####
……..### SWEET. ###
……/….#########…\
..__\…..\..######/…../
(.(.(____)….`.#.´..(____).).)
Gyan Question
Which of the following is not correct
Ans : ( d )
Be, Mg, Ca form mono-oxides while Sr, Ba, Ra form peroxides.
Some content on this page was disabled on October 5, 2016 as a result of a DMCA takedown notice from Reed Elsevier. You can learn more about the DMCA here:
https://wordpress.com/support/copyright-and-the-dmca/
Some content on this page was disabled on July 19, 2017 as a result of a DMCA takedown notice from Pearson Education, Inc.. You can learn more about the DMCA here: